The cell envelope glycoconjugates of Mycobacterium tuberculosis
- PMID: 24915502
- PMCID: PMC4436706
- DOI: 10.3109/10409238.2014.925420
The cell envelope glycoconjugates of Mycobacterium tuberculosis
Abstract
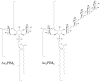
Tuberculosis (TB) remains the second most common cause of death due to a single infectious agent. The cell envelope of Mycobacterium tuberculosis (Mtb), the causative agent of the disease in humans, is a source of unique glycoconjugates and the most distinctive feature of the biology of this organism. It is the basis of much of Mtb pathogenesis and one of the major causes of its intrinsic resistance to chemotherapeutic agents. At the same time, the unique structures of Mtb cell envelope glycoconjugates, their antigenicity and essentiality for mycobacterial growth provide opportunities for drug, vaccine, diagnostic and biomarker development, as clearly illustrated by recent advances in all of these translational aspects. This review focuses on our current understanding of the structure and biogenesis of Mtb glycoconjugates with particular emphasis on one of the most intriguing and least understood aspect of the physiology of mycobacteria: the translocation of these complex macromolecules across the different layers of the cell envelope. It further reviews the rather impressive progress made in the last 10 years in the discovery and development of novel inhibitors targeting their biogenesis.
Keywords: (lipo)polysaccharides; Acyltrehaloses; arabinogalactan; flippase; glycosyltransferase; lipoarabinomannan; peptidoglycan; phosphatidylinositol mannosides.
Figures










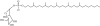


References
-
- AFONSO-BARROSO A, CLARK SO, WILLIAMS A, ROSA GT, NOBREGA C, SILVA-GOMES S, VALE-COSTA S, UMMELS R, STOKER N, MOVAHEDZADEH F, VAN DER LEY P, SLOOTS A, COT M, APPELMELK BJ, PUZO G, NIGOU J, GEURTSEN J, APPELBERG R. Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses. Cell Microbiol. 2012;15:660–674. - PubMed
-
- ALDERWICK LJ, DOVER LG, SEIDEL M, GANDE R, SAHM H, EGGELING L, BESRA GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-D-arabinose metabolism. Glycobiology. 2006b;16:1073–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
