Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade
- PMID: 24915569
- PMCID: PMC4051644
- DOI: 10.1371/journal.pone.0098415
Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade
Abstract
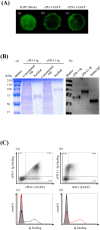
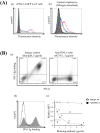
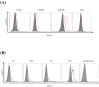
Programmed death 1 (PD-1), an immunoinhibitory receptor, and programmed death ligand 1 (PD-L1), its ligand, together induce the "exhausted" status in antigen-specific lymphocytes and are thus involved in the immune evasion of tumor cells. In this study, canine PD-1 and PD-L1 were molecularly characterized, and their potential as therapeutic targets for canine tumors was discussed. The canine PD-1 and PD-L1 genes were conserved among canine breeds. Based on the sequence information obtained, the recombinant canine PD-1 and PD-L1 proteins were constructed; they were confirmed to bind each other. Antibovine PD-L1 monoclonal antibody effectively blocked the binding of recombinant PD-1 with PD-L1-expressing cells in a dose-dependent manner. Canine melanoma, mastocytoma, renal cell carcinoma, and other types of tumors examined expressed PD-L1, whereas some did not. Interestingly, anti-PD-L1 antibody treatment enhanced IFN-γ production from tumor-infiltrating cells. These results showed that the canine PD-1/PD-L1 pathway is also associated with T-cell exhaustion in canine tumors and that its blockade with antibody could be a new therapeutic strategy for canine tumors. Further investigations are needed to confirm the ability of anti-PD-L1 antibody to reactivate canine antitumor immunity in vivo, and its therapeutic potential has to be further discussed.
Conflict of interest statement
Figures




References
-
- Adams VJ, Evans KM, Sampson J, Wood JL (2010) Methods and mortality results of a health survey of purebred dogs in the UK. J Small Anim Pract 51: 512–524. - PubMed
-
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

