DTAF dye concentrations commonly used to measure microscale deformations in biological tissues alter tissue mechanics
- PMID: 24915570
- PMCID: PMC4051763
- DOI: 10.1371/journal.pone.0099588
DTAF dye concentrations commonly used to measure microscale deformations in biological tissues alter tissue mechanics
Abstract
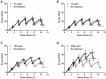
Identification of the deformation mechanisms and specific components underlying the mechanical function of biological tissues requires mechanical testing at multiple levels within the tissue hierarchical structure. Dichlorotriazinylaminofluorescein (DTAF) is a fluorescent dye that is used to visualize microscale deformations of the extracellular matrix in soft collagenous tissues. However, the DTAF concentrations commonly employed in previous multiscale experiments (≥2000 µg/ml) may alter tissue mechanics. The objective of this study was to determine whether DTAF affects tendon fascicle mechanics and if a concentration threshold exists below which any observed effects are negligible. This information is valuable for guiding the continued use of this fluorescent dye in future experiments and for interpreting the results of previous work. Incremental strain testing demonstrated that high DTAF concentrations (≥100 µg/ml) increase the quasi-static modulus and yield strength of rat tail tendon fascicles while reducing their viscoelastic behavior. Subsequent multiscale testing and modeling suggests that these effects are due to a stiffening of the collagen fibrils and strengthening of the interfibrillar matrix. Despite these changes in tissue behavior, the fundamental deformation mechanisms underlying fascicle mechanics appear to remain intact, which suggests that conclusions from previous multiscale investigations of strain transfer are still valid. The effects of lower DTAF concentrations (≤10 µg/ml) on tendon mechanics were substantially smaller and potentially negligible; nevertheless, no concentration was found that did not at least slightly alter the tissue behavior. Therefore, future studies should either reduce DTAF concentrations as much as possible or use other dyes/techniques for measuring microscale deformations.
Conflict of interest statement
Figures






References
-
- Screen HRC, Lee DA, Bader DL, Shelton JC (2004) An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H 218: 109–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

