Conversion to lacosamide monotherapy in the treatment of focal epilepsy: results from a historical-controlled, multicenter, double-blind study
- PMID: 24915838
- PMCID: PMC4477913
- DOI: 10.1111/epi.12681
Conversion to lacosamide monotherapy in the treatment of focal epilepsy: results from a historical-controlled, multicenter, double-blind study
Abstract
Objective: To evaluate the efficacy and safety of conversion to lacosamide 400 mg/day monotherapy in adults with focal epilepsy.
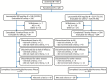
Methods: This historical-controlled, double-blind study (NCT00520741) enrolled patients aged 16-70 years on stable doses of 1-2 antiepileptic drugs (AEDs) and experiencing 2-40 partial-onset seizures per 28 days during the 8-week prospective Baseline. Patients were randomized to lacosamide 400 or 300 mg/day (3:1 ratio), starting at 200 mg/day and titrated over 3 weeks to randomized dose. Patients then withdrew background AEDs over 6 weeks and entered a 10-week Monotherapy Phase. The primary assessment was the Kaplan-Meier-predicted percentage of patients on 400 mg/day in the full analysis set (FAS) meeting ≥ 1 predefined seizure-related exit criterion by day 112, compared with the historical-control threshold (65.3%).
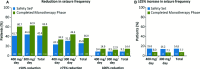
Results: Four hundred twenty-five patients were enrolled and were eligible for safety analyses (400 mg/day, n = 319; 300 mg/day, n = 106). A total of 271 (63.8%) of 425 patients completed the Lacosamide Maintenance Phase (combined AED Withdrawal and Monotherapy Phases). Among 284 patients in the 400 mg/day group in the FAS, 82 (28.9%) met ≥ 1 exit criterion; the Kaplan-Meier-predicted exit percentage at day 112 for 400 mg/day (30.0%; 95% confidence interval [CI] 24.6-35.5%) was lower than the historical control. When exit events, withdrawal due to treatment-emergent adverse events (TEAEs), and withdrawal due to lack of efficacy were summed (n = 90), the predicted exit percentage (32.3%; 95% CI 26.8-37.8%) was also lower than the historical control. Most patients receiving 400 mg/day reported some improvement on the Clinical Global Impression of Change (75.4%) and Patient Global Impression of Change (74.3%). Overall, the most common (>10%) TEAEs were dizziness (24.0%), headache (14.4%), nausea (13.4%), convulsion (11.5%), somnolence (10.4%), and fatigue (10.1%); most (74.1%) were mild-to-moderate in intensity. Seventy-two patients (16.9%) discontinued due to TEAEs. Seventeen patients (4%, all receiving 400 mg/day) experienced serious AEs.
Significance: Lacosamide 400 mg/day monotherapy was effective, with a favorable safety profile in patients with focal epilepsy.
Keywords: Focal epilepsy; Historical control; Lacosamide; Monotherapy; Partial-onset seizures.
Wiley Periodicals, Inc. © 2014 International League Against Epilepsy.
Figures

References
-
- Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9:464–468. - PubMed
-
- Felbatol (felbamate) US prescribing information. Somerset, NJ: MEDA Pharmaceuticals Inc; 2012.
-
- Lamictal (lamotrigine) Summary of product characteristics. Uxbridge, Middlesex, UK: GlaxoSmithKline UK; 2013.
-
- Trileptal (oxcarbazepine) Summary of product characteristics. Camberley, Surrey, UK: Novartis Pharmaceuticals UK Ltd; 2013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

