Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models
- PMID: 24915963
- PMCID: PMC4229780
- DOI: 10.1186/scrt465
Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models
Abstract
Introduction: Mesenchymal stem cell-conditioned medium (MSC-CM) has been shown to have protective effects against various cellular-injury models. This mechanism of protection, however, has yet to be elucidated. Recently, exosomes were identified as the active component in MSC-CM. The aim of this study is to investigate the effect of MSC-derived exosomes in an established carbon tetrachloride (CCl4)-induced liver injury mouse model. This potential effect is then validated by using in vitro xenobiotic-induced liver-injury assays: (1) acetaminophen (APAP)- and (2) hydrogen peroxide (H2O2)-induced liver injury.
Methods: The exosomes were introduced concurrent with CCl4 into a mouse model through different routes of administration. Biochemical analysis was performed based on the blood and liver tissues. Subsequently the exosomes were treated in APAP and H2O2-toxicants with in vitro models. Cell viability was measured, and biomarkers indicative of regenerative and oxidative biochemical responses were determined to probe the mechanism of any hepatoprotective activity observed.
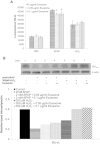
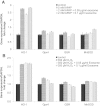
Results: In contrast to mice treated with phosphate-buffered saline, CCl4 injury in mice was attenuated by concurrent-treatment exosomes, and characterized by an increase in hepatocyte proliferation, as demonstrated with proliferating cell nuclear antigen (PCNA) elevation. Significantly higher cell viability was demonstrated in the exosomes-treated group compared with the non-exosome-treated group in both injury models. The higher survival rate was associated with upregulation of the priming-phase genes during liver regeneration, which subsequently led to higher expression of proliferation proteins (PCNA and cyclin D1) in the exosomes-treated group. Exosomes also inhibited the APAP- and H2O2-induced hepatocytes apoptosis through upregulation of Bcl-xL protein expression. However, exosomes do not mitigate hepatocyte injury via modulation of oxidative stress.
Conclusions: In summary, these results suggest that MSC-derived exosomes can elicit hepatoprotective effects against toxicants-induced injury, mainly through activation of proliferative and regenerative responses.
Figures






References
-
- Thorgeirsson SS. Hepatic stem cells in liver regeneration. FASEB J. 1996;10:1249–1256. - PubMed
-
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. - PubMed
-
- Daniels D, Grytdal S, Wasley A. Centers for Disease Control and Prevention: surveillance for acute viral hepatitis, United States, 2007. MMWR Surveill Summ. 2009;58:1–27. - PubMed
-
- Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. - PubMed
-
- Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

