Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan
- PMID: 24915987
- PMCID: PMC4064805
- DOI: 10.1186/1532-429X-16-40
Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan
Abstract
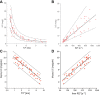
Background: There is a need to standardise non-invasive measurements of liver iron concentrations (LIC) so clear inferences can be drawn about body iron levels that are associated with hepatic and extra-hepatic complications of iron overload. Since the first demonstration of an inverse relationship between biopsy LIC and liver magnetic resonance (MR) using a proof-of-concept T2* sequence, MR technology has advanced dramatically with a shorter minimum echo-time, closer inter-echo spacing and constant repetition time. These important advances allow more accurate calculation of liver T2* especially in patients with high LIC.
Methods: Here, we used an optimised liver T2* sequence calibrated against 50 liver biopsy samples on 25 patients with transfusional haemosiderosis using ordinary least squares linear regression, and assessed the method reproducibility in 96 scans over an LIC range up to 42 mg/g dry weight (dw) using Bland-Altman plots. Using mixed model linear regression we compared the new T2*-LIC with R2-LIC (Ferriscan) on 92 scans in 54 patients with transfusional haemosiderosis and examined method agreement using Bland-Altman approach.
Results: Strong linear correlation between ln(T2*) and ln(LIC) led to the calibration equation LIC = 31.94(T2*)-1.014. This yielded LIC values approximately 2.2 times higher than the proof-of-concept T2* method. Comparing this new T2*-LIC with the R2-LIC (Ferriscan) technique in 92 scans, we observed a close relationship between the two methods for values up to 10 mg/g dw, however the method agreement was poor.
Conclusions: New calibration of T2* against liver biopsy estimates LIC in a reproducible way, correcting the proof-of-concept calibration by 2.2 times. Due to poor agreement, both methods should be used separately to diagnose or rule out liver iron overload in patients with increased ferritin.
Figures



References
-
- Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, Olivieri N, Piga A, Cunningham MJ, Soulieres D, Gattermann N, Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–176. - PMC - PubMed
-
- Aydinok Y, Ulger Z, Nart D, Terzi A, Cetiner N, Ellis G, Zimmermann A, Manz C. A randomized controlled 1-year study of daily deferiprone plus twice weekly desferrioxamine compared with daily deferiprone monotherapy in patients with thalassemia major. Haematologica. 2007;92:1599–1606. doi: 10.3324/haematol.11414. - DOI - PubMed
-
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. - DOI - PubMed
-
- Brittenham GM, Cohen AR, McLaren CE, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol. 1993;42:81–85. doi: 10.1002/ajh.2830420116. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

