TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord
- PMID: 24916269
- PMCID: PMC4384652
- DOI: 10.1007/s00401-014-1299-6
TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord
Abstract
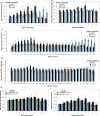
We examined the phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) inclusions as well as neuronal loss in full-length spinal cords and five selected regions of the central nervous system from 36 patients with amyotrophic lateral sclerosis (ALS) and 10 age-matched normal controls. The most severe neuronal loss and pTDP-43 lesions were seen in lamina IX motor nuclei columns 4, 6, and 8 of lower cervical segments and in columns 9-11 of lumbosacral segments. Severity of pTDP-43 pathology and neuronal loss correlated closely with gray and white matter oligodendroglial involvement and was linked to onset of disease, with severe involvement of columns 4, 6, and 8 of upper extremity onset cases and severe involvement of columns of 9, 10, and 11 in cases with lower extremity onset. Severe TDP-43 lesions and neuronal loss were observed in stage 4 cases and sometimes included Onuf's nucleus. Notably, three cases displayed pTDP-43 aggregates in the midbrain oculomotor nucleus, which we had not seen previously even in cases with advanced (i.e., stage 4) pathology. pTDP-43 aggregates were observed in neurons of Clarke's column in 30.6 % of cases but rarely in the intermediolateral nucleus (IML). Gray matter oligodendroglial pTDP-43 inclusions were present in areas devoid of neuronal pTDP-43 aggregates and neuronal loss. Taken together, our findings indicate that (1) the dorsolateral motor nuclei columns of the cervical and lumbosacral anterior horn may be the earliest foci of pTDP-43 pathology in the spinal cord, (2) gray matter oligodendroglial involvement is an early event in the ALS disease process that possibly heralds subsequent involvement of neurons by pTDP-43 pathology, and (3) in some very advanced cases, there is oculomotor nucleus involvement, which may constitute an additional neuropathological stage (designated here as stage 5) of pTDP-43 pathology in ALS.
Figures




References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. - PubMed
-
- Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25:708–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG010124/AG/NIA NIH HHS/United States
- K08 AG039510/AG/NIA NIH HHS/United States
- AG010124/AG/NIA NIH HHS/United States
- K08 AG033101/AG/NIA NIH HHS/United States
- AG039510/AG/NIA NIH HHS/United States
- NS044266/NS/NINDS NIH HHS/United States
- K23 NS088341/NS/NINDS NIH HHS/United States
- R01 NS044266/NS/NINDS NIH HHS/United States
- P01 AG032953/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- AG017586/AG/NIA NIH HHS/United States
- AG032953/AG/NIA NIH HHS/United States
- AG033101/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

