Biophysical characterization of the type III secretion tip proteins and the tip proteins attached to bacterium-like particles
- PMID: 24916512
- PMCID: PMC4262743
- DOI: 10.1002/jps.24047
Biophysical characterization of the type III secretion tip proteins and the tip proteins attached to bacterium-like particles
Abstract
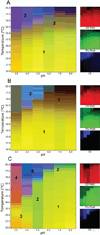
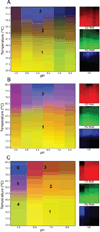
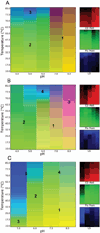
Bacterium-like particles (BLPs), derived from Lactococcus lactis, offer a self-adjuvanting delivery vehicle for subunit protein vaccines. Proteins can be specifically loaded onto the BLPs via a peptidoglycan anchoring (PA) domain. In this study, the tip proteins IpaD, SipD, and LcrV belonging to type III secretion systems of Shigella flexneri, Salmonella enterica, and Yersinia enterocolitica, respectively, were fused to the PA and loaded onto the BLPs. Herein, we biophysically characterized these nine samples and condensed the spectroscopic results into three-index empirical phase diagrams (EPDs). The EPDs show distinctions between the IpaD/SipD and LcrV subfamilies of tip proteins, based on their physical stability, even upon addition of the PA. Upon attachment to the BLPs, the BLPs become defining moiety in the spectroscopic measurements, leaving the tip proteins to have a subtle yet modulating effect on the structural integrity of the tip proteins-BLPs binding. In summary, this work provides a comprehensive view of physical stability of the tip proteins and tip protein-BLPs and serves as a baseline for screening of excipients to increase the stability of the tip protein-BLPs for future vaccine formulation.
Keywords: circular dichroism; fluorescence spectroscopy; light scattering (static); pH; physical characterization; physical stability.
© 2014 Wiley Periodicals, Inc. and the American Pharmacists Association.
Figures
Similar articles
-
Bacterium-like particles derived from probiotics: progress, challenges and prospects.Front Immunol. 2023 Oct 6;14:1263586. doi: 10.3389/fimmu.2023.1263586. eCollection 2023. Front Immunol. 2023. PMID: 37868963 Free PMC article. Review.
-
Biophysical Characterization of the Type III Secretion System Translocator Proteins and the Translocator Proteins Attached to Bacterium-Like Particles.J Pharm Sci. 2015 Dec;104(12):4065-4073. doi: 10.1002/jps.24659. Epub 2015 Sep 30. J Pharm Sci. 2015. PMID: 26422758
-
pH sensitivity of type III secretion system tip proteins.Proteins. 2008 Jun;71(4):1830-42. doi: 10.1002/prot.21864. Proteins. 2008. PMID: 18175320
-
Conformational stability and differential structural analysis of LcrV, PcrV, BipD, and SipD from type III secretion systems.Protein Sci. 2007 Apr;16(4):704-14. doi: 10.1110/ps.062645007. Epub 2007 Feb 27. Protein Sci. 2007. PMID: 17327391 Free PMC article.
-
Förster resonance energy transfer (FRET) as a tool for dissecting the molecular mechanisms for maturation of the Shigella type III secretion needle tip complex.Int J Mol Sci. 2012 Nov 16;13(11):15137-61. doi: 10.3390/ijms131115137. Int J Mol Sci. 2012. PMID: 23203116 Free PMC article. Review.
Cited by
-
Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice.Immunol Cell Biol. 2015 Aug;93(7):641-52. doi: 10.1038/icb.2015.24. Epub 2015 Mar 17. Immunol Cell Biol. 2015. PMID: 25776843 Free PMC article.
-
GEM-PA-Based Subunit Vaccines of Crimean Congo Hemorrhagic Fever Induces Systemic Immune Responses in Mice.Viruses. 2022 Jul 28;14(8):1664. doi: 10.3390/v14081664. Viruses. 2022. PMID: 36016285 Free PMC article.
-
Bacterium-like particles derived from probiotics: progress, challenges and prospects.Front Immunol. 2023 Oct 6;14:1263586. doi: 10.3389/fimmu.2023.1263586. eCollection 2023. Front Immunol. 2023. PMID: 37868963 Free PMC article. Review.
References
-
- Leenhouts K. Mimopath-Based Vaccine Delivery. In: Singh M, editor. Novel Immune Potentiators and Delivery Technologies for Next Generation Vaccines. New York: Springer; 2013. pp. 245–265.
-
- van Roosmalen ML, Kanninga R, El Khattabi M, Neef J, Audouy S, Bosma T, Kuipers A, Post E, Steen A, Kok J, Buist G, Kuipers OP, Robillard G, Leenhouts K. Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods. 2006;38(2):144–149. - PubMed
-
- Ramirez K, Ditamo Y, Rodriguez L, Picking WL, van Roosmalen ML, Leenhouts K, Pasetti MF. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal immunology. 2010;3(2):159–171. - PMC - PubMed
-
- Keijzer CMT, Voorn P, de Haan A, Haijema BJ, Leenhouts K, van Roosmalen ML, van Eden W, Broere F. Inactivated influenza vaccine adjuvanted with bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine. 2013 in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

