Methotrexate for treating rheumatoid arthritis
- PMID: 24916606
- PMCID: PMC7047041
- DOI: 10.1002/14651858.CD000957.pub2
Methotrexate for treating rheumatoid arthritis
Abstract
Background: Methotrexate is a folic acid antagonist widely used for the treatment of neoplastic disorders. Methotrexate inhibits the synthesis of deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and proteins by binding to dihydrofolate reductase. Currently, methotrexate is among the most commonly used drugs for the treatment of rheumatoid arthritis (RA). This is an update of the previous Cochrane systematic review published in 1997.
Objectives: To evaluate short term benefits and harms of methotrexate for treating RA compared to placebo.
Search methods: The Cochrane Musculoskeletal Group Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE were searched from 1966 to 1997 and then updated to November 2013. The search was complemented with a bibliography search of the reference lists of trials retrieved from the electronic search.
Selection criteria: Randomized controlled trials and controlled clinical trials comparing methotrexate (MTX) monotherapy against placebo alone in people with RA. Any trial duration and MTX doses were included.
Data collection and analysis: Two review authors independently determined which studies were eligible for inclusion, extracted data and assessed risk of bias. Outcomes were pooled using mean differences (MDs) for continuous variables or standardized mean differences (SMDs) when different scales were used to measure the same outcome. Pooled risk ratio (RR) was used for dichotomous variables. Fixed-effect models were used throughout, although random-effects models were used for outcomes showing heterogeneity.
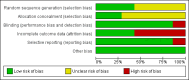
Main results: Five trials with 300 patients were included in the original version of the review. An additional two trials with 432 patients were added to the 2013 update of the review for a total of 732 participants. The trials were generally of unclear to low risk of bias with a follow-up duration ranging from 12 to 52 weeks. All trials included patients who have failed prior treatment (for example, gold therapy, D-penicillamine, azathioprine or anti-malarials); mean disease duration that ranged between 1 and 14 years with six trials reporting more than 4 years; and weekly doses that ranged between 5 mg and 25 mg.
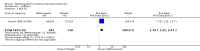
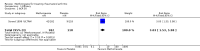
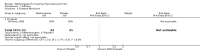
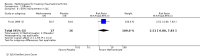
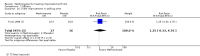
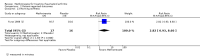
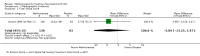
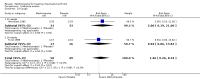
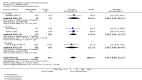
Benefits: Statistically significant and clinically important differences were observed for most efficacy outcomes. MTX monotherapy showed a clinically important and statistically significant improvement in the American College of Rheumatology (ACR) 50 response rate when compared with placebo at 52 weeks (RR 3.0, 95% confidence interval (CI) 1.5 to 6.0; number needed to treat (NNT) 7, 95% CI 4 to 22). Fifteen more patients out of 100 had a major improvement in the ACR 50 outcome compared to placebo (absolute treatment benefit (ATB) 15%, 95% CI 8% to 23%).Statistically significant improvement in physical function (scale of 0 to 3) was also observed in patients receiving MTX alone compared with placebo at 12 to 52 weeks (MD -0.27, 95% CI -0.39 to -0.16; odds ratio (OR) 2.8, 95% CI 0.23 to 32.2; NNT 4, 95% CI 3 to 7). Nine more patients out of 100 improved in physical function compared to placebo (ATB -9%, 95% CI -13% to -5.3%). Similarly, the proportion of patients who improved at least 20% on the Short Form-36 (SF-36) physical component was higher in the MTX-treated group compared with placebo at 52 weeks (RR 1.5, 95% CI 1.0 to 2.1; NNT 9, 95% CI 4 to 539). Twelve more patients out of 100 showed an improvement of at least 20% in the physical component of the quality of life measure compared to placebo (ATB 12%, 95% CI 1% to 24%). No clinically important or statistically significant differences were observed in the SF-36 mental component.Although no statistically significant differences were observed in radiographic scores (that is, Total Sharp score, erosion score, joint space narrowing), radiographic progression rates (measured by an increase in erosion scores of more than 3 units on a scale ranging from 0 to 448) were statistically significantly lower for patients in the MTX group compared with placebo-treated patients (RR 0.31, 95% CI 0.11 to 0.86; NNT 13, 95% CI 10 to 60). Eight more patients out of 100 showed less damage to joints measured by an increase in erosion scores compared to placebo (ATB -8%, 95% CI -16% to -1%). In the one study measuring remission, no participants in either group met the remission criteria. These are defined by at least five of (≥ 2 months): morning stiffness of < 15 minutes, no fatigue, no joint pain by history, no joint tenderness, no joint swelling, and Westergren erythrocyte sedimentation rate (ESR) of < 20 mm/hr in men and < 30 mm/hr in women.
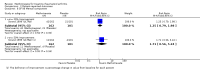
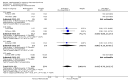
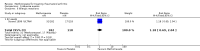
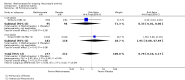
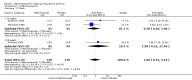
Harms: Patients in the MTX monotherapy group were twice as likely to discontinue from the study due to adverse events compared to patients in the placebo group, at 12 to 52 weeks (16% versus 8%; RR 2.1, 95% CI 1.3 to 3.3; NNT 13, 95% CI 6 to 44). Compared to placebo, nine more people out of 100 who took MTX withdrew from the studies because of side effects (ATB 9%, 95% CI 3% to 14%). Total adverse event rates at 12 weeks were higher in the MTX monotherapy group compared to the placebo group (45% versus 15%; RR 3.0, 95% CI 1.4 to 6.4; NNT 4, 95% CI 2 to 17). Thirty more people out of 100 who took MTX compared to those who took placebo experienced any type of side effect (common or rare) (ATB 30, 95% CI 13% to 47%). No statistically significant differences were observed in the total number of serious adverse events between the MTX group and the placebo group at 27 to 52 weeks. Three people out of 100 who took MTX alone experienced rare but serious side effects compared to 2 people out of 100 who took a placebo (3% versus 2%, respectively).
Authors' conclusions: Based on mainly moderate to high quality evidence, methotrexate (weekly doses ranging between 5 mg and 25 mg) showed a substantial clinical and statistically significant benefit compared to placebo in the short term treatment (12 to 52 weeks) of people with RA, although its use was associated with a 16% discontinuation rate due to adverse events.
Conflict of interest statement
Dr Suarez‐Almazor is the recipient of a K24 career award from the National Institute for Musculoskeletal and Skin Disorders. She is also the Director of the Houston Centre for Education and Research on Therapeutics, funded by the Agency for Healthcare Research and Quality (AHRQ).
Figures








































































Update of
-
Methotrexate for rheumatoid arthritis.Cochrane Database Syst Rev. 2000;(2):CD000957. doi: 10.1002/14651858.CD000957. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2014 Jun 10;(6):CD000957. doi: 10.1002/14651858.CD000957.pub2. PMID: 10796399 Updated.
Comment in
-
Methotrexate therapy for rheumatoid arthritis.Am Fam Physician. 2015 Jan 1;91(1):26-7. Am Fam Physician. 2015. PMID: 25591196 No abstract available.
References
References to studies included in this review
Andersen 1985 {published data only}
-
- Andersen PA, West SG, O'Dell JR, Via CS, Claypool RG, Kotzin BL. Weekly pulse methotrexate in rheumatoid arthritis. Clinical and immunologic effects in a randomized, double‐blind study. Annals of Internal Medicine 1985;103(4):489‐96. - PubMed
Furst 1989 {published data only}
-
- Furst DE, Erikson N, Clute L, Koehnke R, Burmeister LF, Kohler JA. Adverse experience with methotrexate during 176 weeks of a long‐term prospective trial in patients with rheumatoid arthritis. Journal of Rheumatology 1990;17(12):1628‐35. - PubMed
-
- Furst DE, Koehnke R, Burmeister LF, Kohler J, Cargill I. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. Journal of Rheumatology 1989;16(3):313‐20. - PubMed
Jiang 1998 {published data only}
-
- Jiang LD, Wang JY, Mei ZW, Ni LQ. Clinical effectiveness of methotrexate treatment in rheumatoid arthritis patients: a randomized controlled trial. Chinese Journal of Rheumatology 1998;4:204‐7.
Pinheiro 1993 {published data only}
-
- Pinheiro GR, Helfenstein Junior M, Ferraz MB, Atra E. [A short‐term randomized controlled study with methotrexate in rheumatoid arthritis]. [Portuguese]. Revista Da Associacao Medica Brasileira 1993;39(2):91‐4. - PubMed
Strand 1999 (ULTRA) {published data only}
-
- Schiff MH. Leflonomide versus methotrexate: A comparison of the European and American experience. Scandinavian Journal of Rheumatology 1999;28 Suppl 112:31‐5. - PubMed
-
- Sharp JT, Strand V, Leung H, Hurley F, Loew‐Friedrich I. Treatment with leflunomide slows radiographic progression of rheumatoid arthritis: results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis and Rheumatism 2000;43(3):495‐505. [PUBMED: 10728741] - PubMed
-
- Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Archives of Internal Medicine 1999;159(21):2542‐50. [PUBMED: 10573044] - PubMed
-
- Strand V, Tugwell P, Bombardier C, Maetzel A, Crawford B, Dorrier C, et al. Function and health‐related quality of life: results from a randomized controlled trial of leflunomide versus methotrexate or placebo in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis and Rheumatism 1999;42(9):1870‐8. [PUBMED: 10513801] - PubMed
-
- Tugwell P, Wells G, Strand V, Maetzel A, Bombardier C, Crawford B, et al. Clinical improvement as reflected in measures of function and health‐related quality of life following treatment with leflunomide compared with methotrexate in patients with rheumatoid arthritis: sensitivity and relative efficiency to detect a treatment effect in a twelve‐month, placebo‐controlled trial. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis and Rheumatism 43;3:506‐14. [PUBMED: 10728742] - PubMed
Weinblatt 1985 {published data only}
-
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE. Efficacy of low‐dose methotrexate in rheumatoid arthritis. New England Journal of Medicine 1985;312(13):818‐22. - PubMed
Williams 1985 {published data only}
-
- Alarcon GS, Billingsley LM, Clegg DO, Hardin JG, Klippel, Luggen ME, et al. Lack of association between HLA‐DR2 and clinical response to methotrexate in patients with rheumatoid arthritis. Arthritis and Rheumatism 1987;30(2):218‐20. - PubMed
-
- Tugwell P, Bombardier C, Buchanan WW, Goldsmith C, Grace E, Bennett KJ, et al. Methotrexate in rheumatoid arthritis. Impact on quality of life assessed by traditional standard‐item and individualized patient preference health status questionnaires. Archives of Internal Medicine 1990;150(150):59‐62. - PubMed
-
- Williams HJ, Willkens RF, Samuelson CO Jr, Alarcon GS, Guttadauria M, Yarboro C, et al. Comparison of low‐dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis and Rheumatism 1985;28(7):721‐30. - PubMed
References to studies excluded from this review
Cannon 1990 {published data only}
-
- Cannon GW, Reading JC, Ward JR, Blonquist LJ, Collette LB. Clinical and laboratory outcomes during the treatment of rheumatoid arthritis with methotrexate. Scandinavian Journal of Rheumatology 1990;19:285‐94. - PubMed
Cohen 2001 {published data only}
-
- Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. Two‐year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis and Rheumatism 2001;44(9):1984‐92. [PUBMED: 11592358] - PubMed
Jones 2010 (AMBITION) {published data only}
-
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez‐Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Annals of the Rheumatic Diseases 2010;69(1):88‐96. [PUBMED: 19297346] - PMC - PubMed
Szanto 1986 {published data only}
-
- Szanto E. Low‐dose methotrexate in rheumatoid arthritis: effect and tolerance. An open trial and a double‐blind randomized study. Scandinavian Journal of Rheumatology 1986;15:97‐102. - PubMed
Thompson 1984 {published data only}
-
- Thompson RN, Watts C, Edelman J, Esdaile J, Russell AS. A controlled two‐centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. Journal of Rheumatology 1984;11:760‐3. - PubMed
Weinblatt 1998 {published data only}
-
- Weinblatt ME, Maier AL, Fraser PA, Coblyn JS. Longterm prospective study of methotrexate in rheumatoid arthritis: conclusion after 132 months of therapy. Journal of Rheumatology 1998;25(2):238‐42. [PUBMED: 9489813] - PubMed
Additional references
Aga 2013
-
- Aga AB, Lie E, Uhlig T, Olsen IC, Wierød A, Kalstad S, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR‐DMARD study 2000‐2010. Annals of the Rheumatic Diseases 2013;Nov 27:epub. [doi: 10.1136/annrheumdis‐2013‐204020; PUBMED: 24285493] - PubMed
Cates 2008
-
- Visual Rx [Computer program]. Version 3.. Dr. Christopher Cates EBM web site 2008:URL: http://www.nntonline.net.
Dickersin 1994
Felson 1992
-
- Felson DT, Anderson JJ, Meenan RF. Use of short‐term efficacy/toxicity tradeoffs to select second‐line drugs in rheumatoid arthritis. A meta‐analysis of published clinical trials. Arthritis and Rheumatism 1992;35:1117‐25. - PubMed
Felson 1993
-
- Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis and Rheumatism 1993;36:729‐40. - PubMed
Felson 1995
-
- Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38:727‐35. - PubMed
Gotzsche 1992
-
- Gøtzsche PC, Pødenphant J, Olesen M, Halberg P. Meta‐analysis of second‐line antirheumatic drugs: sample size bias and uncertain benefit. Journal of Clinical Epidemiology 1992;45(6):587‐94. [PUBMED: 1535101] - PubMed
Groff 1983
-
- Groff BD, Shenberger KN, Wilke WS, Taylor TH. Low dose oral methotrexate in rheumatoid arthritis: An uncontrolled trial and review of the literature. Seminars in Arthritis and Rheumatism 1983;12:333‐47. - PubMed
Gubner 1951
-
- Gubner R, August S, Ginsburg V. Therapeutic suppression of tissue reactivity.II. Effects of aminopterin in rheumatoid arthritis and psoriasis. American Journal of Medicine 1951;221(2):176‐82. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration 2011;Available from www.cochrane‐handbook.org.:Accessed on: 09/16/2013.
Jadad 1996
-
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kapral 2006
Kazis 1989
-
- Kazis LEE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Medical Care 1989;27(S3):S178‐89. - PubMed
Mueller 2007
-
- Mueller PS1, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomised trials early because of apparent benefit. Annals of Internal Medicine 2007;146(12):878‐81. [PUBMED: 17577007] - PubMed
OMERACT 1993
-
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Journal of Rheumatology 1993;20:526‐91. - PubMed
Petitti 1994
-
- Petitti D. Meta‐analysis, decision analysis, and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. Meta‐analysis, decision analysis, and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994:90‐114.
Shea 2003
-
- Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2013;5(CD000951):doi: 10.1002/14651858.CD000951.pub2.. [PUBMED: 23728635] - PMC - PubMed
Shea 2013
-
- Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2013;5:CD000951. [DOI: 10.1002/14651858.CD000951.pub2] - DOI - PMC - PubMed
Singh 2012
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care and Research (Hoboken) 2012;64(5):625‐39. - PMC - PubMed
Smolen 2013
-
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 2013;Oct 25:doi: 10.1136/annrheumdis‐2013‐204573. - PMC - PubMed
Steinsson 1982
-
- Steinsson K, Weinstein A, Korn J, Abeles M. Low dose methotrexate in rheumatoid arthritis. Journal of Rheumatology 1982;9:860‐6. - PubMed
Todoerti 2013
-
- Todoerti M, Maglione W, Bernero E, Bortoluzzi A, Colaci M, Galuppi E, et al. Systematic review of 2008‐2012 literature and update of recommendations for the use of methotrexate in rheumatic diseases, with a focus on rheumatoid arthritis. Reumatismo 2013;65(5):207‐18. [PUBMED: 24399184] - PubMed
van der Heijde 2005
van der Heijde 2007
-
- Heijde D, Klareskog L, Landewé R, Bruyn GA, Cantagrel A, Durez P, et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis and Rheumatism 2007;56(1):3928‐39. [PUBMED: 18050208] - PubMed
Weinstein 1985
-
- Weinstein A, Marlowe S, Korn J, Farouhar F. Low‐dose methotrexate treatment of rheumatoid arthritis. Long‐term observations. American Journal of Medicine 1985;79(3):331‐7. [PUBMED: 4036984] - PubMed
Willkens 1980
-
- Willkens RF, Watson MA, Paxson CS. Low dose pulse methotrexate therapy in rheumatoid arthritis. Journal of Rheumatology 1980;7:501‐5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

