Excited state potential energy surfaces and their interactions in Fe(IV)=O active sites
- PMID: 24916844
- PMCID: PMC4229428
- DOI: 10.1039/c4dt01366b
Excited state potential energy surfaces and their interactions in Fe(IV)=O active sites
Abstract
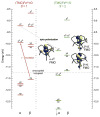
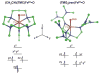
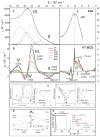
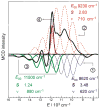
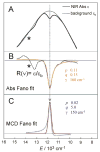
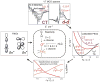
The non-heme ferryl active sites are of significant interest for their application in biomedical and green catalysis. These sites have been shown to have an S = 1 or S = 2 ground spin state; the latter is functional in biology. Low-temperature magnetic circular dichroism (LT MCD) spectroscopy probes the nature of the excited states in these species including ligand-field (LF) states that are otherwise difficult to study by other spectroscopies. In particular, the temperature dependences of MCD features enable their unambiguous assignment and thus determination of the low-lying excited states in two prototypical S = 1 and S = 2 NHFe(IV)[double bond, length as m-dash]O complexes. Furthermore, some MCD bands exhibit vibronic structures that allow mapping of excited-state interactions and their effects on the potential energy surfaces (PESs). For the S = 2 species, there is also an unusual spectral feature in both near-infrared absorption and MCD spectra - Fano antiresonance (dip in Abs) and Fano resonance (sharp peak in MCD) that indicates the weak spin-orbit coupling of an S = 1 state with the S = 2 LF state. These experimental data are correlated with quantum-chemical calculations that are further extended to analyze the low-lying electronic states and the evolution of their multiconfigurational characters along the Fe-O PESs. These investigations show that the lowest-energy states develop oxyl Fe(III) character at distances that are relevant to the transition state (TS) for H-atom abstraction and define the frontier molecular orbitals that participate in the reactivity of S = 1 vs. S = 2 non-heme Fe(IV)[double bond, length as m-dash]O active sites. The S = 1 species has only one available channel that requires the C-H bond of a substrate to approach perpendicular to the Fe-oxo bond (the π channel). In contrast, there are three channels (one σ and two π) available for the S = 2 non-heme Fe(IV)[double bond, length as m-dash]O system allowing C-H substrate approach both along and perpendicular to the Fe-oxo bond that have important implications for enzymatic selectivity.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

