Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors
- PMID: 24916856
- PMCID: PMC4140984
- DOI: 10.1002/glia.22703
Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors
Abstract
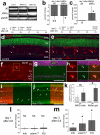
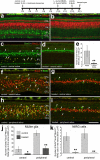
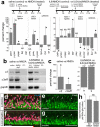
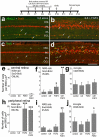
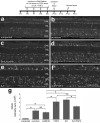
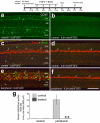
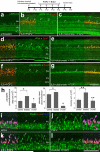
In retinas where Müller glia have been stimulated to become progenitor cells, reactive microglia are always present. Thus, we investigated how the activation or ablation of microglia/macrophage influences the formation of Müller glia-derived progenitor cells (MGPCs) in the retina in vivo. Intraocular injections of the Interleukin-6 (IL6) stimulated the reactivity of microglia/macrophage, whereas other types of retinal glia appear largely unaffected. In acutely damaged retinas where all of the retinal microglia/macrophage were ablated, the formation of proliferating MGPCs was greatly diminished. With the microglia ablated in damaged retinas, levels of Notch and related genes were unchanged or increased, whereas levels of ascl1a, TNFα, IL1β, complement component 3 (C3) and C3a receptor were significantly reduced. In the absence of retinal damage, the combination of insulin and Fibroblast growth factor 2 (FGF2) failed to stimulate the formation of MGPCs when the microglia/macrophage were ablated. In addition, intraocular injections of IL6 and FGF2 stimulated the formation of MGPCs in the absence of retinal damage, and this generation of MGPCs was blocked when the microglia/macrophage were absent. We conclude that the activation of microglia and/or infiltrating macrophage contributes to the formation of proliferating MGPCs, and these effects may be mediated by components of the complement system and inflammatory cytokines.
Keywords: Müller glia; cellular proliferation; microglia; regeneration; retina.
© 2014 Wiley Periodicals, Inc.
Figures


References
-
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001;13(6):706–14. - PubMed
-
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25(3):277–95. - PubMed
-
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24(2):161–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

