Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis
- PMID: 24916964
- PMCID: PMC4296347
- DOI: 10.1002/ana.24195
Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis
Abstract
Objective: A severe but treatable form of immune-mediated encephalitis is associated with antibodies in serum and cerebrospinal fluid (CSF) against the GluN1 subunit of the N-methyl-D-aspartate receptor (NMDAR). Prolonged exposure of hippocampal neurons to antibodies from patients with anti-NMDAR encephalitis caused a reversible decrease in the synaptic localization and function of NMDARs. However, acute effects of the antibodies, fate of the internalized receptors, type of neurons affected, and whether neurons develop compensatory homeostatic mechanisms were unknown and are the focus of this study.
Methods: Dissociated hippocampal neuron cultures and rodent brain sections were used for immunocytochemical, physiological, and molecular studies.
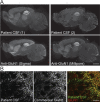
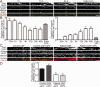
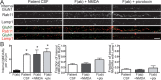
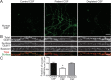
Results: Patient antibodies bind to NMDARs throughout the rodent brain, and decrease NMDAR cluster density in both excitatory and inhibitory hippocampal neurons. They rapidly increase the internalization rate of surface NMDAR clusters, independent of receptor activity. This internalization likely accounts for the observed decrease in NMDAR-mediated currents, as no evidence of direct blockade was detected. Once internalized, antibody-bound NMDARs traffic through both recycling endosomes and lysosomes, similar to pharmacologically induced NMDAR endocytosis. The antibodies are responsible for receptor internalization, as their depletion from CSF abrogates these effects in hippocampal neurons. We find that although anti-NMDAR antibodies do not induce compensatory changes in glutamate receptor gene expression, they cause a decrease in inhibitory synapse density onto excitatory hippocampal neurons.
Interpretation: Our data support an antibody-mediated mechanism of disease pathogenesis driven by immunoglobulin-induced receptor internalization. Antibody-mediated downregulation of surface NMDARs engages homeostatic synaptic plasticity mechanisms, which may inadvertently contribute to disease progression.
© 2014 The Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures



References
-
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

