The vascular steal phenomenon is an incomplete contributor to negative cerebrovascular reactivity in patients with symptomatic intracranial stenosis
- PMID: 24917040
- PMCID: PMC4158662
- DOI: 10.1038/jcbfm.2014.106
The vascular steal phenomenon is an incomplete contributor to negative cerebrovascular reactivity in patients with symptomatic intracranial stenosis
Abstract
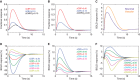
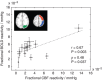
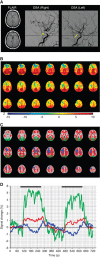
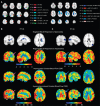
'Vascular steal' has been proposed as a compensatory mechanism in hemodynamically compromised ischemic parenchyma. Here, independent measures of cerebral blood flow (CBF) and blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) responses to a vascular stimulus in patients with ischemic cerebrovascular disease are recorded. Symptomatic intracranial stenosis patients (n=40) underwent a multimodal 3.0T MRI protocol including structural (T1-weighted and T2-weighted fluid-attenuated inversion recovery) and hemodynamic (BOLD and CBF-weighted arterial spin labeling) functional MRI during room air and hypercarbic gas administration. CBF changes in regions demonstrating negative BOLD reactivity were recorded, as well as clinical correlates including symptomatic hemisphere by infarct and lateralizing symptoms. Fifteen out of forty participants exhibited negative BOLD reactivity. Of these, a positive relationship was found between BOLD and CBF reactivity in unaffected (stenosis degree<50%) cortex. In negative BOLD cerebrovascular reactivity regions, three patients exhibited significant (P<0.01) reductions in CBF consistent with vascular steal; six exhibited increases in CBF; and the remaining exhibited no statistical change in CBF. Secondary findings were that negative BOLD reactivity correlated with symptomatic hemisphere by lateralizing clinical symptoms and prior infarcts(s). These data support the conclusion that negative hypercarbia-induced BOLD responses, frequently assigned to vascular steal, are heterogeneous in origin with possible contributions from autoregulation and/or metabolism.
Figures


References
-
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124 (Pt 3:457–467. - PubMed
-
- Gao YZ, Zhang JJ, Liu H, Wu GY, Xiong L, Shu M. Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer's disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr Neurovasc Res. 2013;10:49–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

