Mitigation of oxygen-induced retinopathy in α2β1 integrin-deficient mice
- PMID: 24917135
- PMCID: PMC4102391
- DOI: 10.1167/iovs.14-14061
Mitigation of oxygen-induced retinopathy in α2β1 integrin-deficient mice
Abstract
Purpose: The α2β1 integrin plays an important but complex role in angiogenesis and vasculopathies. Published GWAS studies established a correlation between genetic polymorphisms of the α2β1 integrin gene and incidence of diabetic retinopathy. Recent studies indicated that α2-null mice demonstrate superior vascularization in both the wound and diabetic microenvironments. The goal of this study was to determine whether the vasculoprotective effects of α2-integrin deficiency extended to the retina, using the oxygen-induced retinopathy (OIR) model for retinopathy of prematurity (ROP).
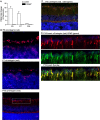
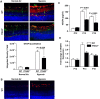
Methods: In the OIR model, wild-type (WT) and α2-null mice were exposed to 75% oxygen for 5 days (postnatal day [P] 7 to P12) and subsequently returned to room air for 6 days (P12-P18). Retinas were collected at postnatal day 7, day 13, and day 18 and examined via hematoxylin and eosin and Lectin staining. Retinas were analyzed for retinal vascular area, neovascularization, VEGF expression, and Müller cell activation. Primary Müller cell cultures from WT and α2-null mice were isolated and analyzed for hypoxia-induced VEGF-A expression.
Results: In the retina, the α2β1 integrin was minimally expressed in endothelial cells and strongly expressed in activated Müller cells. Isolated α2-null primary Müller cells demonstrated decreased hypoxia-induced VEGF-A expression. In the OIR model, α2-null mice displayed reduced hyperoxia-induced vaso-attenuation, reduced pathological retinal neovascularization, and decreased VEGF expression as compared to WT counterparts.
Conclusions: Our data suggest that the α2β1 integrin contributes to the pathogenesis of retinopathy. We describe a newly identified role for α2β1 integrin in mediating hypoxia-induced Müller cell VEGF-A production.
Keywords: Müller cells; angiogenesis; integrin; retinopathy.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures



References
-
- Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999; 3: 26–32 - PubMed
-
- Bressler NM, Bressler SB. Preventative ophthalmology. Age-related macular degeneration. Ophthalmology. 1995; 102: 1206–1211 - PubMed
-
- Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996; 103: 1721–1726 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

