Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos
- PMID: 24917499
- PMCID: PMC4050694
- DOI: 10.1242/dev.105346
Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos
Abstract
A common feature of development in most vertebrate models is the early segregation of the germ line from the soma. For example, in Xenopus and zebrafish embryos primordial germ cells (PGCs) are specified by germ plasm that is inherited from the egg; in mice, Blimp1 expression in the epiblast mediates the commitment of cells to the germ line. How these disparate mechanisms of PGC specification evolved is unknown. Here, in order to identify the ancestral mechanism of PGC specification in vertebrates, we studied PGC specification in embryos from the axolotl (Mexican salamander), a model for the tetrapod ancestor. In the axolotl, PGCs develop within mesoderm, and classic studies have reported their induction from primitive ectoderm (animal cap). We used an axolotl animal cap system to demonstrate that signalling through FGF and BMP4 induces PGCs. The role of FGF was then confirmed in vivo. We also showed PGC induction by Brachyury, in the presence of BMP4. These conditions induced pluripotent mesodermal precursors that give rise to a variety of somatic cell types, in addition to PGCs. Irreversible restriction of the germ line did not occur until the mid-tailbud stage, days after the somatic germ layers are established. Before this, germline potential was maintained by MAP kinase signalling. We propose that this stochastic mechanism of PGC specification, from mesodermal precursors, is conserved in vertebrates.
Keywords: Axolotl; Evolution; Germ plasm; Mesoderm; PGC; Pluripotency; Primordial germ cell.
© 2014. Published by The Company of Biologists Ltd.
Figures
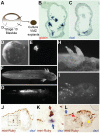

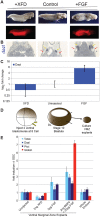
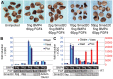
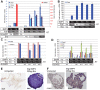


References
-
- Aramaki S., Hayashi K., Kurimoto K., Ohta H., Yabuta Y., Iwanari H., Mochizuki Y., Hamakubo T., Kato Y., Shirahige K., et al. (2013). A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev. Cell 27, 516-529 10.1016/j.devcel.2013.11.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

