Notch signaling functions in lymphatic valve formation
- PMID: 24917500
- PMCID: PMC4050693
- DOI: 10.1242/dev.101188
Notch signaling functions in lymphatic valve formation
Abstract
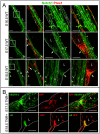
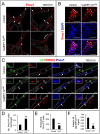
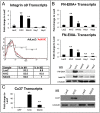
Collecting lymphatic ducts contain intraluminal valves that prevent backflow. In mice, lymphatic valve morphogenesis begins at embryonic day 15.5 (E15.5). In the mesentery, Prox1 expression is high in valve-forming lymphatic endothelial cells, whereas cells of the lymphatic ducts express lower levels of Prox1. Integrin α9, fibronectin EIIIA, Foxc2, calcineurin and the gap junction protein Cx37 are required for lymphatic valve formation. We show that Notch1 is expressed throughout the developing mesenteric lymphatic vessels at E16.5, and that, by E18.5, Notch1 expression becomes highly enriched in the lymphatic valve endothelial cells. Using a Notch reporter mouse, Notch activity was detected in lymphatic valves at E17.5 and E18.5. The role of Notch in lymphatic valve morphogenesis was studied using a conditional lymphatic endothelial cell driver either to delete Notch1 or to express a dominant-negative Mastermind-like (DNMAML) transgene. Deletion of Notch1 led to an expansion of Prox1(high) cells, a defect in Prox1(high) cell reorientation and a decrease in integrin α9 expression at sites of valve formation. Expression of DNMAML, which blocks all Notch signaling, resulted in a more severe phenotype characterized by a decrease in valves, failure of Prox1(high) cells to cluster, and rounding of the nuclei and decreased fibronectin-EIIIA expression in the Prox1(high) cells found at valve sites. In human dermal lymphatic endothelial cells, activation of Notch1 or Notch4 induced integrin α9, fibronectin EIIIA and Cx37 expression. We conclude that Notch signaling is required for proper lymphatic valve formation and regulates integrin α9 and fibronectin EIIIA expression during valve morphogenesis.
Keywords: Integrin α9; Lymphatic valve morphogenesis; Mouse; Notch.
© 2014. Published by The Company of Biologists Ltd.
Figures

References
-
- Kanady J. D., Dellinger M. T., Munger S. J., Witte M. H., Simon A. M. (2011). Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 354, 253-266 10.1016/j.ydbio.2011.04.004 - DOI - PMC - PubMed
-
- Kang J., Yoo J., Lee S., Tang W., Aguilar B., Ramu S., Choi I., Out H. H., Shin J. W., Dotto G. P., Koh C. J., Detmar M., Hong Y. K. (2010). An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood 116, 140-150 10.2353/ajpath.2008.080378 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

