Novel calmodulin mutations associated with congenital arrhythmia susceptibility
- PMID: 24917665
- PMCID: PMC4140998
- DOI: 10.1161/CIRCGENETICS.113.000459
Novel calmodulin mutations associated with congenital arrhythmia susceptibility
Abstract
Background: Genetic predisposition to life-threatening cardiac arrhythmias such as congenital long-QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) represent treatable causes of sudden cardiac death in young adults and children. Recently, mutations in calmodulin (CALM1, CALM2) have been associated with severe forms of LQTS and CPVT, with life-threatening arrhythmias occurring very early in life. Additional mutation-positive cases are needed to discern genotype-phenotype correlations associated with calmodulin mutations.
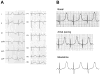
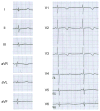
Methods and results: We used conventional and next-generation sequencing approaches, including exome analysis, in genotype-negative LQTS probands. We identified 5 novel de novo missense mutations in CALM2 in 3 subjects with LQTS (p.N98S, p.N98I, p.D134H) and 2 subjects with clinical features of both LQTS and CPVT (p.D132E, p.Q136P). Age of onset of major symptoms (syncope or cardiac arrest) ranged from 1 to 9 years. Three of 5 probands had cardiac arrest and 1 of these subjects did not survive. The clinical severity among subjects in this series was generally less than that originally reported for CALM1 and CALM2 associated with recurrent cardiac arrest during infancy. Four of 5 probands responded to β-blocker therapy, whereas 1 subject with mutation p.Q136P died suddenly during exertion despite this treatment. Mutations affect conserved residues located within Ca(2+)-binding loops III (p.N98S, p.N98I) or IV (p.D132E, p.D134H, p.Q136P) and caused reduced Ca(2+)-binding affinity.
Conclusions: CALM2 mutations can be associated with LQTS and with overlapping features of LQTS and CPVT.
Keywords: calmodulin; long QT syndrome.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures






References
-
- Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, et al. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation. 1995;92:2929–2934. - PubMed
-
- Zhang L, Timothy KW, Vincent GM, Lehmann MH, Fox J, Giuli LC, et al. Spectrum of ST-T-wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation. 2000;102:2849–2855. - PubMed
-
- Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. - PubMed
-
- Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

