Mechanism of ATPase-mediated Cu+ export and delivery to periplasmic chaperones: the interaction of Escherichia coli CopA and CusF
- PMID: 24917681
- PMCID: PMC4110264
- DOI: 10.1074/jbc.M114.577668
Mechanism of ATPase-mediated Cu+ export and delivery to periplasmic chaperones: the interaction of Escherichia coli CopA and CusF
Abstract
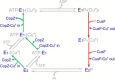
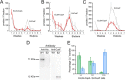
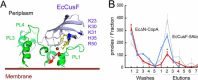
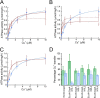
Cellular copper homeostasis requires transmembrane transport and compartmental trafficking while maintaining the cell essentially free of uncomplexed Cu(2+/+). In bacteria, soluble cytoplasmic and periplasmic chaperones bind and deliver Cu(+) to target transporters or metalloenzymes. Transmembrane Cu(+)-ATPases couple the hydrolysis of ATP to the efflux of cytoplasmic Cu(+). Cytosolic Cu(+) chaperones (CopZ) interact with a structural platform in Cu(+)-ATPases (CopA) and deliver copper into the ion permeation path. CusF is a periplasmic Cu(+) chaperone that supplies Cu(+) to the CusCBA system for efflux to the extracellular milieu. In this report, using Escherichia coli CopA and CusF, direct Cu(+) transfer from the ATPase to the periplasmic chaperone was observed. This required the specific interaction of the Cu(+)-bound form of CopA with apo-CusF for subsequent metal transfer upon ATP hydrolysis. As expected, the reverse Cu(+) transfer from CusF to CopA was not observed. Mutation of CopA extracellular loops or the electropositive surface of CusF led to a decrease in Cu(+) transfer efficiency. On the other hand, mutation of Met and Glu residues proposed to be part of the metal exit site in the ATPase yielded enzymes with lower turnover rates, although Cu(+) transfer was minimally affected. These results show how soluble chaperones obtain Cu(+) from transmembrane transporters. Furthermore, by explaining the movement of Cu(+) from the cytoplasmic pool to the extracellular milieu, these data support a mechanism by which cytoplasmic Cu(+) can be precisely directed to periplasmic targets via specific transporter-chaperone interactions.
Keywords: Chaperone; Copper; Copper ATPase; Copper Transport; Metal Homeostasis; Metal Ion-Protein Interaction.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Fraústo da Silva J. J. R., Williams R. J. P. (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd Ed., Oxford University Press, Oxford
-
- Rensing C., McDevitt S. F. (2013) The copper metallome in prokaryotic cells. Met. Ions Life Sci. 12, 417–450 - PubMed
-
- Valko M., Morris H., Cronin M. T. (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

