Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis
- PMID: 24917940
- PMCID: PMC4040783
- DOI: 10.1177/2050640612474651
Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis
Abstract
Background: Prucalopride is a selective, high-affinity, 5-hydroxytryptamine (serotonin) type 4 (5-HT4) receptor agonist with gastrointestinal prokinetic activities. This integrated analysis of data from three double-blind phase III trials (ClinicalTrials.gov: NCT00488137, NCT00483886, NCT00485940) compared the efficacy and safety of prucalopride 2 mg once daily in women with chronic constipation [≤2 spontaneous complete bowel movements (SCBM) per week] in whom laxatives had failed to provide adequate relief with that in the all-patient (AP) population of men and women with chronic constipation who had or had not obtained relief from laxatives.
Methods: Patients received prucalopride 2 mg or placebo once-daily for 12 weeks. Efficacy endpoints included an average of ≥3 SCBM/week and average increases of ≥1 SCBM/week and ≥1 SBM/week over this period. A response on any of these three endpoints was considered to be clinically relevant, and an overall response rate was derived for patients satisfying any of these endpoints.
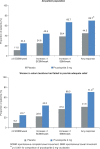
Results: Of the AP population (n = 1318), 936 were women in whom laxatives had failed to provide adequate relief (WLF). More patients on prucalopride 2 mg than placebo had an average of ≥3 SCBM/week (AP 24.4 vs 11.0%; WLF 24.7 vs 9.2%), an average increase of ≥1 SCBM/week (AP 43.5 vs 24.8%; WLF 44.2 vs 22.6%), and an average increase of ≥1 SBM/week (AP 66.7 vs 38.4%; WLF 68.3 vs 37.0%) (all p < 0.001). Significant differences from placebo were evident in week 1 and sustained thereafter. Overall response rates in the AP and WLF populations, respectively, were 69.7 and 71.0% with prucalopride 2 mg and 44.5 and 41.6% with placebo (p < 0.001). Early (weeks 1-4) response predicted ultimate response over time. Common (>10%) adverse events were abdominal pain, nausea, diarrhoea, and headache.
Conclusions: Prucalopride 2 mg once daily is effective in WLF. The efficacy and safety profile observed in WLF was similar to that in the total evaluated population of patients with chronic constipation who had or had not obtained adequate relief from laxatives.
Keywords: 5-HT4 agonist; efficacy; safety; serotonergic.
Figures



References
-
- World Gastroenterology Organisation. World Gastroenterology Organisation practice guidelines: constipation. Available at: www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/05_cons... (2007)
-
- Locke 3rd, GR, Pemberton JH, Phillips SF. American Gastroenterological Association medical position statement: guidelines on constipation. Gastroenterology 2000; 119: 1761–1766 - PubMed
-
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005; 100(Suppl. 1): S1–S4 - PubMed
-
- Rome Foundation. Rome III diagnostic criteria for functional gastrointestinal disorders. Available at: www.romecriteria.org/assets/pdf/19_RomeIII_apA_885–898.pdf (2006) - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

