Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer
- PMID: 24918061
- PMCID: PMC4050189
- DOI: 10.1016/j.fob.2014.04.007
Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer
Abstract
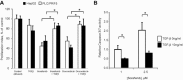
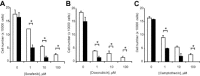
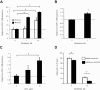
Hepatocellular cancers (HCC) are highly resistant to chemotherapy. TGFβ has been associated with chemoresistance in some human cancers but the mechanisms involved are unknown. We explored how TGFβ might contribute to altered responses to therapy by assessing the involvement and mechanistic contribution of extracellular vesicle long non-coding RNA (lncRNA) in mediating TGFβ-dependent chemoresistance. TGFβ reduced the sensitivity of HCC cells to sorafenib or doxorubicin and altered the release of both extracellular vesicles and of selected lncRNA within these vesicles. Amongst these, lincRNA-ROR (linc-ROR), a stress-responsive lncRNA was highly expressed in HCC cells and enriched within extracellular vesicles derived from tumor cells. Incubation with HCC-derived extracellular vesicles increased linc-ROR expression and reduced chemotherapy-induced cell death in recipient cells. Sorafenib increased linc-ROR expression in both tumor cells and extracellular vesicles, whereas siRNA to linc-ROR increased chemotherapy-induced apoptosis and cytotoxicity. Tumor-initiating cells that express CD133 have an increased resistance to therapy. TGFβ increased expression of CD133+ cells and colony growth in limiting dilution assays, both of which were attenuated by linc-ROR knockdown. These data provide mechanistic insights into primary chemoresistance in HCC by showing that: (a) TGFβ selectively enriches linc-RoR within extracellular vesicles, which has a potential role in intercellular signaling in response to TGFβ; (b) expression and enrichment of linc-ROR during chemotherapeutic stress plays a functional role in chemoresistance; and (c) the effects of TGFβ on chemoresistance in HCC may involve linc-RoR-dependent effects on tumor-initiating cells. These findings implicate extracellular vesicle lncRNA as mediators of the chemotherapeutic response, and support targeting linc-ROR to enhance chemosensitivity in HCC.
Keywords: CT, cycle threshold; Chemoresistance; EV, extracellular vesicle; Exosomes; Gene expression; HCC, hepatocellular carcinoma; Liver cancer; RNA genes; TGFβ, transforming growth factor β; VD, vesicle-depleted; linc-ROR, long intergenic non-coding RNA; lncRNA, long non-coding RNA; miRNA, microRNA; siRNA, small interfering RNA.
Figures




References
-
- El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. - PubMed
-
- Asghar U., Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J. Hepatol. 2012;56:686–695. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

