Synthesis of isochromene-type scaffolds via single-flask Diels-Alder-[4 + 2]-annulation sequence of a silyl-substituted diene with menadione
- PMID: 24918110
- PMCID: PMC4076005
- DOI: 10.1021/ol501329t
Synthesis of isochromene-type scaffolds via single-flask Diels-Alder-[4 + 2]-annulation sequence of a silyl-substituted diene with menadione
Abstract
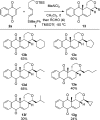
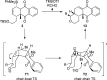
A sequential Diels-Alder reaction/silicon-directed [4 + 2]-annulation was developed to assemble hydroisochromene-type ring systems from menadione 2. In the first step, a Diels-Alder of the 1-silyl-substituted butadiene 1 with 2 furnished an intermediate cyclic allylsilane. Subsequently, TMSOTf promoted a [4 + 2]-annulation through trapping of an oxonium, generated by condensation between an aldehyde and the TBS protected alcohol resulted in the formation of a cis-fused hydroisochromene 13.
Figures


Similar articles
-
Boron and Silicon-Substituted 1,3-Dienes and Dienophiles and Their Use in Diels-Alder Reactions.Molecules. 2020 Aug 16;25(16):3740. doi: 10.3390/molecules25163740. Molecules. 2020. PMID: 32824327 Free PMC article. Review.
-
Silyl-substituted 1,3-butadienes for Diels-Alder reaction, ene reaction and allylation reaction.Chem Commun (Camb). 2011 Apr 21;47(15):4348-57. doi: 10.1039/c0cc05665k. Epub 2011 Feb 21. Chem Commun (Camb). 2011. PMID: 21336391 Review.
-
Synthesis of (+)-bullatacin via the highly diastereoselective [3+2] annulation reaction of a racemic aldehyde and a nonracemic allylsilane.Org Lett. 2005 Sep 15;7(19):4245-8. doi: 10.1021/ol051719k. Org Lett. 2005. PMID: 16146398 Free PMC article.
-
Enantioselective synthesis of allenylenol silyl ethers via chiral lithium amide mediated reduction of ynenoyl silanes and their Diels-Alder reactions.Org Lett. 2015 Mar 6;17(5):1280-3. doi: 10.1021/acs.orglett.5b00261. Epub 2015 Feb 17. Org Lett. 2015. PMID: 25689472
-
Temporary silicon connection strategies in intramolecular allylation of aldehydes with allylsilanes.J Org Chem. 2008 Jul 18;73(14):5462-75. doi: 10.1021/jo800824x. Epub 2008 Jun 17. J Org Chem. 2008. PMID: 18557649
Cited by
-
Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer's Disease.Pharmaceuticals (Basel). 2021 Jan 5;14(1):33. doi: 10.3390/ph14010033. Pharmaceuticals (Basel). 2021. PMID: 33466332 Free PMC article. Review.
-
Boron and Silicon-Substituted 1,3-Dienes and Dienophiles and Their Use in Diels-Alder Reactions.Molecules. 2020 Aug 16;25(16):3740. doi: 10.3390/molecules25163740. Molecules. 2020. PMID: 32824327 Free PMC article. Review.
-
Palladium mediated domino reaction: synthesis of isochromenes under aqueous medium.RSC Adv. 2020 Jan 2;10(1):338-349. doi: 10.1039/c9ra08792c. eCollection 2019 Dec 20. RSC Adv. 2020. PMID: 35492569 Free PMC article.
References
-
-
For ventiloquinones, see:
- Hanumaiah T.; Marshall D. S.; Rao B. K.; Rao C. P.; Rao G. S. R.; Rao J. U. M.; Rao K. V. L; Thomson R. H. Phytochemistry 1985, 24, 2373–2378.
- Jammula S. R.; Papalla S. B.; Telikepalli H.; Rao K. V. J.; Thomson R. H. Phytochemistry 1991, 30, 3741–3744.
- Ali S.; Read R. W.; Sotheeswaran S. Phytochemistry 1994, 35, 1029–1032.
- Brimble M. A.; Dancalf L. J.; Nairn M. R. Nat. Prod. Rep. 1999, 16, 267–281. - PubMed
-
For other examples of natural products and bioactive molecules, see
- Hayashi T.; Smith F. T.; Lee K.-H. J. Med. Chem. 1987, 30, 2005–2008. - PubMed
- Abas F.; Lajis N. H.; Shaari K.; Israf D. A.; Stanslas J.; Yusuf U. K.; Raof S. M. J. Nat. Prod. 2005, 68, 1090–1093. - PubMed
- Shishido Y.; Wakabayashi H.; Koike H.; Ueno N.; Nukui S.; Yamagishi T.; Murata Y.; Naganeo F.; Mizutani M.; Shimada K.; Fujiwara Y.; Sakakibara A.; Suga O.; Kusano R.; Ueda S.; Kanai Y.; Tsuchiya M.; Satake K. Bioorg. Med. Chem. 2008, 16, 7193–7205. - PubMed
- Kuo Y.-J.; Hsiao P.-C.; Zhang L.-J.; Wu M.-D.; Liang Y.-H.; Ho H.-O.; Kuo Y.-H. J. Nat. Prod. 2009, 72, 1097–1101. - PubMed
-
-
- Butin A. V.; Abaev V. T.; Mel’chin V. V.; Dmitriev A. S. Tetrahedron Lett. 2005, 46, 8439–8441.
- Villeneuve K.; Tam W. Eur. J. Org. Chem. 2006, 5449–5453.
- Obika S.; Kono H.; Yasui Y.; Yanada R.; Takemoto Y. J. Org. Chem. 2007, 72, 4462–4468. - PubMed
- Morimoto K.; Hirano K.; Satoh T.; Miura M. J. Org. Chem. 2011, 76, 9548–9551. - PubMed
- Murai M.; Uenishi J.; Uemura M. Org. Lett. 2010, 12, 4788–4791. - PubMed
- Murai M.; Sota Y.; Onohara Y.; Uenishi J.; Uemura M. J. Org. Chem. 2013, 78, 10986–10995. - PubMed
- Malhotra D.; Liu L.-P.; Mashuta M. S.; Hammond G. B. Chem.—Eur. J. 2013, 19, 4043–4050. - PubMed
- Saito K.; Kajiwara Y.; Akiyama T. Angew. Chem., Int. Ed. 2013, 52, 13284–13288. - PubMed
- Cui Y.; Villafane L. A.; Clausen D. J.; Floreancig P. E. Tetrahedron 2013, 69, 7618–7626. - PMC - PubMed
-
- Yadav J. S.; Reddy B. V. S.; Ganesh A. V.; Kumar G. G. K. S. N. Tetrahedron Lett. 2010, 51, 2963–2966.
- Reddy B. V. S.; Borkar P.; Yadav J. S.; Sridhar B.; Grée R. J. Org. Chem. 2011, 76, 7677–7690. - PubMed
- Reddy B. V. S.; Kumar H.; Borkar P.; Yadav J. S.; Sridhar B. Eur. J. Org. Chem. 2013, 1993–1999.
-
- Wu J.; Pu Y.; Panek J. S. J. Am. Chem. Soc. 2012, 134, 18440–18446. - PubMed
-
- Huang H.; Panek J. S. J. Am. Chem. Soc. 2000, 122, 9836–9837.
- Lowe J. T.; Panek J. S. Org. Lett. 2005, 7, 3231–3234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

