Synthesis of site-specific DNA-protein conjugates and their effects on DNA replication
- PMID: 24918113
- PMCID: PMC4136702
- DOI: 10.1021/cb5001795
Synthesis of site-specific DNA-protein conjugates and their effects on DNA replication
Abstract
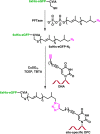
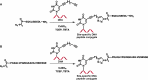
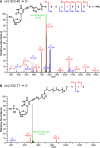
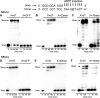
DNA-protein cross-links (DPCs) are bulky, helix-distorting DNA lesions that form in the genome upon exposure to common antitumor drugs, environmental/occupational toxins, ionizing radiation, and endogenous free-radical-generating systems. As a result of their considerable size and their pronounced effects on DNA-protein interactions, DPCs can interfere with DNA replication, transcription, and repair, potentially leading to mutagenesis, genotoxicity, and cytotoxicity. However, the biological consequences of these ubiquitous lesions are not fully understood due to the difficulty of generating DNA substrates containing structurally defined, site-specific DPCs. In the present study, site-specific cross-links between the two biomolecules were generated by copper-catalyzed [3 + 2] Huisgen cycloaddition (click reaction) between an alkyne group from 5-(octa-1,7-diynyl)-uracil in DNA and an azide group within engineered proteins/polypeptides. The resulting DPC substrates were subjected to in vitro primer extension in the presence of human lesion bypass DNA polymerases η, κ, ν, and ι. We found that DPC lesions to the green fluorescent protein and a 23-mer peptide completely blocked DNA replication, while the cross-link to a 10-mer peptide was bypassed. These results indicate that the polymerases cannot read through the larger DPC lesions and further suggest that proteolytic degradation may be required to remove the replication block imposed by bulky DPC adducts.
Figures



References
-
- Barker S.; Weinfeld M.; Murray D. (2005) DNA-protein cross-links: their induction, repair, and biological consequences. Mutat. Res. 589, 111–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

