Assessing subunit dependency of the Plasmodium proteasome using small molecule inhibitors and active site probes
- PMID: 24918547
- PMCID: PMC4136710
- DOI: 10.1021/cb5001263
Assessing subunit dependency of the Plasmodium proteasome using small molecule inhibitors and active site probes
Abstract
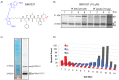
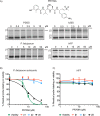
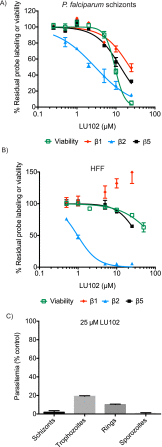
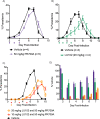
The ubiquitin-proteasome system (UPS) is a potential pathway for therapeutic intervention for pathogens such as Plasmodium, the causative agent of malaria. However, due to the essential nature of this proteolytic pathway, proteasome inhibitors must avoid inhibition of the host enzyme complex to prevent toxic side effects. The Plasmodium proteasome is poorly characterized, making rational design of inhibitors that induce selective parasite killing difficult. In this study, we developed a chemical probe that labels all catalytic sites of the Plasmodium proteasome. Using this probe, we identified several subunit selective small molecule inhibitors of the parasite enzyme complex. Treatment with an inhibitor that is specific for the β5 subunit during blood stage schizogony led to a dramatic decrease in parasite replication while short-term inhibition of the β2 subunit did not affect viability. Interestingly, coinhibition of both the β2 and β5 catalytic subunits resulted in enhanced parasite killing at all stages of the blood stage life cycle and reduced parasite levels in vivo to barely detectable levels. Parasite killing was achieved with overall low host toxicity, something that has not been possible with existing proteasome inhibitors. Our results highlight differences in the subunit dependency of the parasite and human proteasome, thus providing a strategy for development of potent antimalarial drugs with overall low host toxicity.
Figures

References
-
- World Health Organization. World Malaria Report 2011; World Health Organization: Geneva; 2011.
-
- Liu L.; Johnson H. L.; Cousens S.; Perin J.; Scott S.; Lawn J. E.; Rudan I.; Campbell H.; Cibulskis R.; Li M.; Mathers C.; Black R. E. (2012) Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161. - PubMed
-
- Voges D.; Zwickl P.; Baumeister W. (1999) The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68, 1015–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

