Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice
- PMID: 24918580
- PMCID: PMC4053315
- DOI: 10.1371/journal.pone.0096969
Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice
Abstract
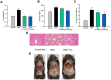
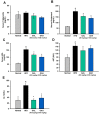
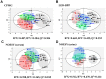
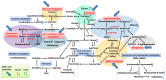
Gallic acid (GA), a naturally abundant plant phenolic compound in vegetables and fruits, has been shown to have potent anti-oxidative and anti-obesity activity. However, the effects of GA on nonalcoholic fatty liver disease (NAFLD) are poorly understood. In this study, we investigated the beneficial effects of GA administration on nutritional hepatosteatosis model by a more "holistic view" approach, namely 1H NMR-based metabolomics, in order to prove efficacy and to obtain information that might lead to a better understanding of the mode of action of GA. Male C57BL/6 mice were placed for 16 weeks on either a normal chow diet, a high fat diet (HFD, 60%), or a high fat diet supplemented with GA (50 and 100 mg/kg/day, orally). Liver histopathology and serum biochemical examinations indicated that the daily administration of GA protects against hepatic steatosis, obesity, hypercholesterolemia, and insulin resistance among the HFD-induced NAFLD mice. In addition, partial least squares discriminant analysis scores plots demonstrated that the cluster of HFD fed mice is clearly separated from the normal group mice plots, indicating that the metabolic characteristics of these two groups are distinctively different. Specifically, the GA-treated mice are located closer to the normal group of mice, indicating that the HFD-induced disturbances to the metabolic profile were partially reversed by GA treatment. Our results show that the hepatoprotective effect of GA occurs in part through a reversing of the HFD caused disturbances to a range of metabolic pathways, including lipid metabolism, glucose metabolism (glycolysis and gluconeogenesis), amino acids metabolism, choline metabolism and gut-microbiota-associated metabolism. Taken together, this study suggested that a 1H NMR-based metabolomics approach is a useful platform for natural product functional evaluation. The selected metabolites are potentially useful as preventive action biomarkers and could also be used to help our further understanding of the effect of GA in hepatosteatosis mice.
Conflict of interest statement
Figures



References
-
- Cusi K (2009) Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 16: 141–149. - PubMed
-
- Maheshwari DT, Yogendra Kumar MS, Verma SK, Singh VK, Singh SN (2011) Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol 49: 2422–2428. - PubMed
-
- Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, et al. (2011) Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem 59: 2663–2671. - PubMed
-
- Wang SH, Kao MY, Wu SC, Lo DY, Wu JY, et al. (2011) Oral administration of Trapa taiwanensis Nakai fruit skin extracts conferring hepatoprotection from CCl4-caused injury. J Agric Food Chem 59: 3686–3692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

