Prostaglandin E2 promotes features of replicative senescence in chronically activated human CD8+ T cells
- PMID: 24918932
- PMCID: PMC4053423
- DOI: 10.1371/journal.pone.0099432
Prostaglandin E2 promotes features of replicative senescence in chronically activated human CD8+ T cells
Erratum in
- PLoS One. 2014;9(9):e107600
Abstract
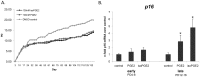
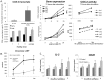
Prostaglandin E2 (PGE2), a pleiotropic immunomodulatory molecule, and its free radical catalyzed isoform, iso-PGE2, are frequently elevated in the context of cancer and chronic infection. Previous studies have documented the effects of PGE2 on the various CD4+ T cell functions, but little is known about its impact on cytotoxic CD8+ T lymphocytes, the immune cells responsible for eliminating virally infected and tumor cells. Here we provide the first demonstration of the dramatic effects of PGE2 on the progression of human CD8+ T cells toward replicative senescence, a terminal dysfunctional state associated multiple pathologies during aging and chronic HIV-1 infection. Our data show that exposure of chronically activated CD8+ T cells to physiological levels of PGE2 and iso-PGE2 promotes accelerated acquisition of markers of senescence, including loss of CD28 expression, increased expression of p16 cell cycle inhibitor, reduced telomerase activity, telomere shortening and diminished production of key cytotoxic and survival cytokines. Moreover, the CD8+ T cells also produced higher levels of reactive oxygen species, suggesting that the resultant oxidative stress may have further enhanced telomere loss. Interestingly, we observed that even chronic activation per se resulted in increased CD8+ T cell production of PGE2, mediated by higher COX-2 activity, thus inducing a negative feedback loop that further inhibits effector function. Collectively, our data suggest that the elevated levels of PGE2 and iso-PGE2, seen in various cancers and HIV-1 infection, may accelerate progression of CD8+ T cells towards replicative senescence in vivo. Inhibition of COX-2 activity may, therefore, provide a strategy to counteract this effect.
Conflict of interest statement
Figures



References
-
- Hammarstrom S (1983) Leukotrienes. Annu Rev Biochem 52: 355–377. - PubMed
-
- Samuelsson B, Goldyne M, Granstrom E, Hamberg M, Hammarstrom S, et al. (1978) Prostaglandins and thromboxanes. Annu Rev Biochem 47: 997–1029. - PubMed
-
- Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, et al. (2004) Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol 11: 328–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

