Dynamic pathways of -1 translational frameshifting
- PMID: 24919156
- PMCID: PMC4472451
- DOI: 10.1038/nature13428
Dynamic pathways of -1 translational frameshifting
Abstract
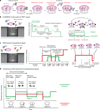
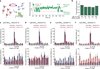
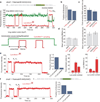
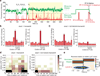
Spontaneous changes in the reading frame of translation are rare (frequency of 10(-3) to 10(-4) per codon), but can be induced by specific features in the messenger RNA (mRNA). In the presence of mRNA secondary structures, a heptanucleotide 'slippery sequence' usually defined by the motif X XXY YYZ, and (in some prokaryotic cases) mRNA sequences that base pair with the 3' end of the 16S ribosomal rRNA (internal Shine-Dalgarno sequences), there is an increased probability that a specific programmed change of frame occurs, wherein the ribosome shifts one nucleotide backwards into an overlapping reading frame (-1 frame) and continues by translating a new sequence of amino acids. Despite extensive biochemical and genetic studies, there is no clear mechanistic description for frameshifting. Here we apply single-molecule fluorescence to track the compositional and conformational dynamics of individual ribosomes at each codon during translation of a frameshift-inducing mRNA from the dnaX gene in Escherichia coli. Ribosomes that frameshift into the -1 frame are characterized by a tenfold longer pause in elongation compared to non-frameshifted ribosomes, which translate through unperturbed. During the pause, interactions of the ribosome with the mRNA stimulatory elements uncouple EF-G catalysed translocation from normal ribosomal subunit reverse-rotation, leaving the ribosome in a non-canonical intersubunit rotated state with an exposed codon in the aminoacyl-tRNA site (A site). tRNA(Lys) sampling and accommodation to the empty A site and EF-G action either leads to the slippage of the tRNAs into the -1 frame or maintains the ribosome into the 0 frame. Our results provide a general mechanistic and conformational framework for -1 frameshifting, highlighting multiple kinetic branchpoints during elongation.
Figures










References
-
- Jenner LB, Demeshkina N, Yusupova G, Yusupov M. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nature structural & molecular biology. 2010;17:555–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

