Trastuzumab-containing regimens for metastatic breast cancer
- PMID: 24919460
- PMCID: PMC6464904
- DOI: 10.1002/14651858.CD006242.pub2
Trastuzumab-containing regimens for metastatic breast cancer
Abstract
Background: Patients with breast cancer are classified as having cells that over-express the human epidermal growth factor receptor 2 (known as HER2-positive) or not (HER2-negative). Typically, patients with HER2-positive disease have a worse prognosis. Trastuzumab is a selective treatment that targets the HER2 pathway. The available evidence supporting trastuzumab regimens mostly relies upon surrogate endpoints and, although the efficacy results seem to support its use, other uncertainties have been raised about its net benefit in relation to transient cardiac toxicity and a long-term increased risk of metastasis to the central nervous system.
Objectives: To assess the evidence on the efficacy and safety of therapy with trastuzumab (overall) and in relation to the type of co-administered regimen and the line of treatment, i.e. first-line or beyond progression, in women with HER2-positive metastatic breast cancer.
Search methods: We searched the Cochrane Breast Cancer Group's (CBCG) Specialised Register and used the search strategy developed by the CBCG to search for randomised controlled trials (RCTs) in CENTRAL (2013, Issue 1), MEDLINE, EMBASE, BIOSIS, the WHO International Clinical Trials Registry Platform (ICTRP) search portal and ClinicalTrials.gov (up to 17 January 2013).
Selection criteria: RCTs comparing the efficacy and safety of trastuzumab alone or in combination with chemotherapy, hormonal therapy or targeted agents in women with HER2-positive metastatic breast cancer.
Data collection and analysis: We collected data from published trials. We used hazard ratios (HRs) for time-to-event outcomes and risk ratio (RRs) for binary outcomes. Subgroup analyses included type of regimen (taxane-containing, anthracycline-containing, aromatase inhibitor-containing or other) and treatment line (first-line, beyond progression).
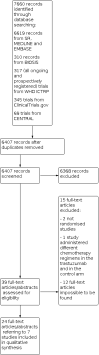
Main results: The review found seven trials, involving 1497 patients, which met the criteria to be included. The trials were generally of moderate methodological quality; two studies have not published their results on overall survival so the presence of selective outcome reporting bias cannot be ruled out. None of the studies used blinding to treatment allocation, though this is unlikely to have biased the results for overall survival. Studies varied in terms of co-administered regimen and in terms of treatment line. In four studies, trastuzumab was administered with a chemotherapy, such as a taxane-containing, anthracycline-containing or capecitabine-containing regimen. Two studies considered postmenopausal women and administered trastuzumab with hormone-blocking medications, such as an aromatase inhibitor. One study administered trastuzumab in addition to lapatinib. Five studies out of seven included women treated with trastuzumab administered until progression as first-line treatment and two studies considered trastuzumab beyond progression. The combined HRs for overall survival and progression-free survival favoured the trastuzumab-containing regimens (HR 0.82, 95% confidence interval (CI) 0.71 to 0.94, P = 0.004; and HR 0.61, 95% CI 0.54 to 0.70, P < 0.00001, respectively; moderate-quality evidence). Trastuzumab increased the risk of congestive heart failure (RR 3.49, 90% CI 1.88 to 6.47, P = 0.0009; moderate-quality evidence) and left ventricular ejection fraction (LVEF) decline (RR 2.65, 90% CI 1.48 to 4.74, P = 0.006). For haematological toxicities, such as neutropenic fever and anaemia, there was no clear evidence that risks differed between groups, while trastuzumab seemed to raise the risk of neutropenia. The overall survival improvement was maintained when considering patients treated as first-line or patients receiving taxane-based regimens. The progression-free survival improvement was maintained when considering patients receiving taxane-based regimens, and patients treated as first-line or subsequent lines. Few data were collected on central nervous system progression. Similarly, few studies reported on quality of life and treatment-related deaths.
Authors' conclusions: Trastuzumab improved overall survival and progression-free survival in HER2-positive women with metastatic breast cancer, but it also increased the risk of cardiac toxicities, such as congestive heart failure and LVEF decline. The available subgroup analyses are limited by the small number of studies. Studies that administered trastuzumab as first-line treatment, or along with a taxane-based regimen, improved mortality outcomes. The evidence to support the use of trastuzumab beyond progression is limited. The recruitment in three out of seven studies was stopped early and in three trials more than 50% of patients in the control groups were permitted to switch to the trastuzumab arms at progression, making it more difficult to understand the real net benefit of trastuzumab.Trastuzumab is generally used for women with HER2-positive early breast cancer in clinical practice, while women enrolled in most of the trials in the metastatic setting were naive to trastuzumab. The effectiveness of trastuzumab for women relapsing after adjuvant trastuzumab is therefore still an open issue, although it is likely that the majority are being offered it again.
Conflict of interest statement
SB: nothing to declare.
SM: nothing to declare.
VG: she acted as speaker for GlaxoSmithkline, and as consultant for Novartis and AstraZeneca. VG institution received a grant from Roche to support a trial in which she is Principal Investigator.
LT: nothing to declare.
VP: nothing to declare.
LM: nothing to declare.
RD: nothing to declare.
Figures
































Comment in
-
Treating metastatic breast cancer: the evidence for targeted therapy.Cochrane Database Syst Rev. 2014 Jun 12;2014(6):ED000083. doi: 10.1002/14651858.ED000083. Cochrane Database Syst Rev. 2014. PMID: 25032250 Free PMC article. No abstract available.
References
References to studies included in this review
Blackwell 2010 {published data only}
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2–positive metastatic breast cancer: final results from the EGF104900 Study. Journal of Clinical Oncology 2012;30(21):2585-92. - PubMed
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. Journal of Clinical Oncology 2010;28(7):1124-30. - PubMed
-
- O'Shaughnessy J, Blackwell KL, Burstein H, Storniolo AM, Sledge G, Baselga J, et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [Abstract 1015]. In: American Society of Clinical Oncology. 2008.
-
- Wu Y, Amonkar MM, Sherrill BH, O'Shaughnessy J, Ellis C, Baselga J, et al. Impact of lapatinib plus trastuzumab versus single agent lapatinib on quality of life of patients with trastuzumab-refractory HER2+ metastatic breast cancer. Annals of Oncology 2011;22(12):2582–90. - PubMed
Gasparini 2006 {published data only}
-
- Gasparini G, Gion M, Crivellari D, Morabito A, Rocco S, Spada A, et al. Interim analysis of a randomized phase IIb study of weekly paclitaxel (PCT) with or without trastuzumab (T) as first-line therapy of patients (pts) with HER-2/neu positive metastatic breast cancer (MBC): Clinical and biological results [Abstract 138]. In: American Society of Clinical Oncology. 2003.
-
- Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Research and Treatment 2007;101(3):355-65. - PubMed
Huober 2012 {published data only}
-
- Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - Results of the eLEcTRA trial. The Breast 2012;21(1):27-33. - PubMed
Kaufman 2009 {published data only}
-
- Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. Journal of Clinical Oncology 2009;27(33):5529-37. - PubMed
Marty 2005 {published data only}
-
- Extra J, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Long-term survival demonstrated with trastuzumab plus docetaxel: 24-month data from a randomised trial (M77001) in HER2-positive metastatic breast cancer [Abstract 555]. In: American Society of Clinical Oncology. 2005.
-
- Extra JM, Cognetti F, Chan S, Maraninchi D, Snyder R, Lluch A, Tubiana-Hulin M, et al. Randomised phase II trial (M77001) of trastuzumab (Herceptin) plus docetaxel versus docetaxel alone, as first-line therapy in patients with HER2-positive metastatic breast cancer. European Journal of Cancer 2003;1:Abstract 672.
-
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. Journal of Clinical Oncology 2005;23(19):4265-74. - PubMed
Slamon 2001 {published data only}
-
- Baselga J, Kerrigan M, Burchmore M, Ash M. Health-related quality of life (HRQL) in women with HER2-overexpressing metastatic breast cancer (MBC) in a phase III study of Herceptin (R) plus chemotherapy versus chemotherapy alone. European Journal of Cancer 1999;35:Abstract 1276.
-
- Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P. Isolated central nervous system metastases in patients withHER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Annals of Oncology 2005;16(11):1772–7. - PubMed
-
- Eiermann W, on behalf of the International Herceptin Study Group. Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: pivotal trial data. Annals of Oncology 2001;12(1):57-62. - PubMed
-
- Osoba D, Burchmore M. Health-related quality of life in women with metastatic breast cancer treated with trastuzumab (Herceptin) [Abstract]. In: Seminars in Oncology. Vol. 26. 1999. - PubMed
-
- Osoba D, Slamon DJ, Burchmore M, Murphy M. Effects of treatment with Her2mab (trastuzumab/Herceptin™) plus chemotherapy (H+C) versus chemotherapy alone (C) on health-related quality of life (HRQL) in women with HER-2/neu-overexpressing metastatic breast cancer [Abstract 109]. In: American Society of Clinical Oncology. 2001.
von Minckwitz 2009 {published data only}
-
- Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. Journal of Clinical Oncology 2009;27(12):1999-2006. - PubMed
-
- Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. European Journal of Cancer 2011;47(15):2273-81. - PubMed
-
- Minckwitz G, Zielinski C, Maarteense E, Vogel P, Schmidt M, Eidtmann H, et al. Capecitabine vs capecitabine+trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: The TBP phase III study (GBG 26/BIG 3-05) [Abstract 1025]. In: American Society of Clinical Oncology. 2008.
References to studies excluded from this review
Gelmon 2012 {published data only}
-
- Gelmon KA, Boyle F, Kaufman B, Huntsman D, Manikhas A, Di Leo A, et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA.31/GSK EGF 108919. Journal of Clinical Oncology 2012;30 Suppl:Abstract LBA 671.
Papaldo 2006 {published data only}
-
- Papaldo P, Fabi A, Ferretti G, Mottolese M, Cianciulli AM, Di Cocco B, et al. A phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: changing the natural history of HER2-positive disease. Annals of Oncology 2006;17(4):630-6. [PMID: ] - PubMed
Raff 2004 {published data only}
-
- Raff JP, Rajdev L, Malik U, Novik Y, Manalo JM, Negassa A, et al. Phase II study of weekly docetaxel alone or in combination with trastuzumab in patients with metastatic breast cancer. Clinical Breast Cancer 2004;4(6):420-7. [PMID: ] - PubMed
Additional references
Apolone 2005
Baselga 1996
-
- Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Journal of Clinical Oncology 1996;14(3):737-44. - PubMed
Bonifazi 2013
Burstein 2005
-
- Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Annals of Oncology 2005;16(11):1772-7. - PubMed
Buzdar 2013
-
- Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al, American College of Surgeons Oncology Group investigators. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. The Lancet Oncology 2013;14(13):1317-25. - PMC - PubMed
Cobleigh 1999
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. Journal of Clinical Oncology 1999;17(9):2639-48. - PubMed
Coussens 1985
-
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 1985;230(4730):1132-9. - PubMed
D'Amico 2011
-
- D'Amico R, Bonafede E, Balduzzi S, Longo G, Guarneri V, Piacentini F, et al. Unplanned crossover in randomized controlled trials: consequences for efficacy and safety outcomes. In: Abstracts of the 19th Cochrane Colloquium; 2011 October 19-22; Madrid. Chichester: John Wiley & Sons, 2011.
Deeks 2003
-
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technology Assessment 2003;7(27):iii-x, 1-173. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
FDA 1998
-
- U.S. Food and Drug Administration. FDA Oncology Tools Approval Summary for Trastuzumab for HERCEPTIN. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed 5 Aug 2013).
Ferlay 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893–917. - PubMed
Fleeman 2011
-
- Fleeman N, Bagust A, Boland A, Dickson R, Dundar Y, Moonan M, et al. Lapatinib and trastuzumab in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptor-positive breast cancer which over-expresses human epidermal growth factor 2(HER2): a systematic review and economic analysis. Health Technology Assessment NIHR HTA programme 2011;15(42):1-93. [DOI: 10.3310/hta15420] - DOI - PMC - PubMed
Gschwind 2004
-
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature Reviews Cancer 2004;4(5):361–70. - PubMed
Guarneri 2012
-
- Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. Journal of Clinical Oncology 2012;30(16):1989-95. - PubMed
Guyatt 2008
Harris 2011
-
- Harris CA, Ward L, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Annals of Oncology 2011;22(6):1308-17. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Ioannidis 2010
-
- Ioannidis JP, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ 2010;341:c4875. [PMID: ] - PubMed
Joppi 2005
Kennecke 2010
-
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology 2010;28(20):3271-7. - PubMed
Liao 2011
-
- Liao C, Yin F, Huang P, Cao Y, Gao F. A meta-analysis of randomised controlled trials comparing chemotherapy plus trastuzumab with chemotherapy alone in HER-2-positive advanced breast cancer. The Breast Journal 2011;17(1):109-11. - PubMed
Mannocci 2010
-
- Mannocci A, De Feo E, Waure C, Specchia ML, Gualano MR, Barone C, et al. Use of trastuzumab in HER2-positive metastatic breast cancer beyond progression: a systematic review of published studies. Tumori 2010;96(3):385-91. - PubMed
Mauri 2008
-
- Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. Journal of the National Cancer Institute 2008;100(24):1780-91. [PMID: ] - PubMed
Miller 2007
-
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. New England Journal of Medicine 2007;357(26):2666-76. - PubMed
Moja 2012
NICE 2002
-
- National Institute for Health and Care Excellence. Breast cancer - trastuzumab [TA 34]. London: National Institute for Health and Care Excellence (http://www.nice.org.uk/page.aspx?o=29274) (accessed 9 February 2006).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Pegram 2004
-
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. Journal of the National Cancer Institute 2004;96(10):739-49. - PubMed
Pestalozzi 2006
-
- Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Annals of Oncology 2006;17:935-44. - PubMed
Schneeweiss 2013
-
- Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Annals of Oncology 2013;9:2278-84. - PubMed
Seidman 2002
-
- Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of Clinical Oncology 2002;20(5):1215-21. - PubMed
Shadish 2002
-
- Shadish WR, Cook TD, Campbell DT. Statistical conclusion validity and internal validity. In: Experimental and Quasi-experimental Designs for Generalized Causal Inference. Boston: Houghton Mifflin Company, 2002.
Simes 2001
-
- Simes RJ, Coates AS. Patient preferences for adjuvant chemotherapy of early breast cancer: how much benefit is needed? Journal of the National Cancer Institute. Monographs 2001;30:146-52. [PMID: ] - PubMed
Slamon 1987
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177-82. - PubMed
Tabouret 2012
-
- Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Research 2012;32(11):4655-62. - PubMed
Vogel 2001
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line Herceptin monotherapy in metastatic breast cancer. Oncology 2001;61 Suppl 2:37-42. - PubMed
Wagner 2012
-
- Wagner AD, Thomssen C, Haerting J, Unverzagt S. Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD008941. [DOI: 10.1002/14651858.CD008941.pub2] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

