Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells
- PMID: 24919951
- PMCID: PMC4095694
- DOI: 10.1186/bcr3675
Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells
Abstract
Introduction: Resistance to anti-estrogen therapies is a major cause of disease relapse and mortality in estrogen receptor alpha (ERα)-positive breast cancers. Tamoxifen or estrogen withdrawal increases the dependence of breast cancer cells on Notch signalling. Here, we investigated the contribution of Nicastrin and Notch signalling in endocrine-resistant breast cancer cells.
Methods: We used two models of endocrine therapies resistant (ETR) breast cancer: tamoxifen-resistant (TamR) and long-term estrogen-deprived (LTED) MCF7 cells. We evaluated the migratory and invasive capacity of these cells by Transwell assays. Expression of epithelial to mesenchymal transition (EMT) regulators as well as Notch receptors and targets were evaluated by real-time PCR and western blot analysis. Moreover, we tested in vitro anti-Nicastrin monoclonal antibodies (mAbs) and gamma secretase inhibitors (GSIs) as potential EMT reversal therapeutic agents. Finally, we generated stable Nicastrin overexpessing MCF7 cells and evaluated their EMT features and response to tamoxifen.
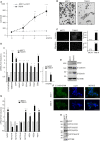
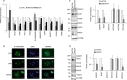
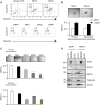
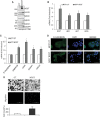
Results: We found that ETR cells acquired an epithelial to mesenchymal transition (EMT) phenotype and displayed increased levels of Nicastrin and Notch targets. Interestingly, we detected higher level of Notch4 but lower levels of Notch1 and Notch2 suggesting a switch to signalling through different Notch receptors after acquisition of resistance. Anti-Nicastrin monoclonal antibodies and the GSI PF03084014 were effective in blocking the Nicastrin/Notch4 axis and partially inhibiting the EMT process. As a result of this, cell migration and invasion were attenuated and the stem cell-like population was significantly reduced. Genetic silencing of Nicastrin and Notch4 led to equivalent effects. Finally, stable overexpression of Nicastrin was sufficient to make MCF7 unresponsive to tamoxifen by Notch4 activation.
Conclusions: ETR cells express high levels of Nicastrin and Notch4, whose activation ultimately drives invasive behaviour. Anti-Nicastrin mAbs and GSI PF03084014 attenuate expression of EMT molecules reducing cellular invasiveness. Nicastrin overexpression per se induces tamoxifen resistance linked to acquisition of EMT phenotype. Our finding suggest that targeting Nicastrin and/or Notch4 warrants further clinical evaluation as valid therapeutic strategies in endocrine-resistant breast cancer.
Figures

Similar articles
-
Essential role of Notch4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer.Cancer Lett. 2017 Apr 1;390:115-125. doi: 10.1016/j.canlet.2017.01.014. Epub 2017 Jan 18. Cancer Lett. 2017. PMID: 28108315
-
Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4-Dependent Cancer Stem Cell Activity.Cell Rep. 2015 Sep 29;12(12):1968-77. doi: 10.1016/j.celrep.2015.08.050. Epub 2015 Sep 17. Cell Rep. 2015. PMID: 26387946 Free PMC article.
-
Biological and clinical implications of nicastrin expression in invasive breast cancer.Breast Cancer Res Treat. 2011 Jan;125(1):43-53. doi: 10.1007/s10549-010-0823-1. Epub 2010 Mar 12. Breast Cancer Res Treat. 2011. PMID: 20224929
-
Notch Signaling in Breast Cancer: A Role in Drug Resistance.Cells. 2020 Sep 29;9(10):2204. doi: 10.3390/cells9102204. Cells. 2020. PMID: 33003540 Free PMC article. Review.
-
Mammary stem cells and breast cancer--role of Notch signalling.Stem Cell Rev. 2007 Jun;3(2):169-75. doi: 10.1007/s12015-007-0023-5. Stem Cell Rev. 2007. PMID: 17873349 Review.
Cited by
-
Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4.BMC Cancer. 2015 Jul 24;15:540. doi: 10.1186/s12885-015-1561-x. BMC Cancer. 2015. PMID: 26206152 Free PMC article.
-
Drug Efflux Transporters Are Overexpressed in Short-Term Tamoxifen-Induced MCF7 Breast Cancer Cells.Adv Pharmacol Sci. 2016;2016:6702424. doi: 10.1155/2016/6702424. Epub 2016 Feb 14. Adv Pharmacol Sci. 2016. PMID: 26981116 Free PMC article.
-
The Kraken Wakes: induced EMT as a driver of tumour aggression and poor outcome.Clin Exp Metastasis. 2018 Apr;35(4):285-308. doi: 10.1007/s10585-018-9906-x. Epub 2018 Jun 8. Clin Exp Metastasis. 2018. PMID: 29948647 Review.
-
The complex nature of heterogeneity and its roles in breast cancer biology and therapeutic responsiveness.Front Endocrinol (Lausanne). 2023 Feb 23;14:1083048. doi: 10.3389/fendo.2023.1083048. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36909339 Free PMC article. Review.
-
Moving Breast Cancer Therapy up a Notch.Front Oncol. 2018 Nov 20;8:518. doi: 10.3389/fonc.2018.00518. eCollection 2018. Front Oncol. 2018. PMID: 30515368 Free PMC article. Review.
References
-
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. - PMC - PubMed
-
- Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R, Barrow D, Nicholson RI. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. - PubMed
-
- Hutcheson IR, Knowlden JM, Madden TA, Barrow D, Gee JM, Wakeling AE, Nicholson RI. Oestrogen receptor-mediated modulation of the EGFR/MAPK pathway in tamoxifen-resistant MCF-7 cells. Breast Cancer Res Treat. 2003;81:81–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

