Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice
- PMID: 24919967
- PMCID: PMC4107738
- DOI: 10.1093/brain/awu140
Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice
Abstract
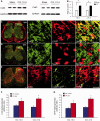
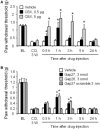
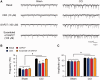
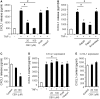
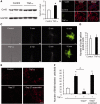
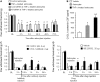
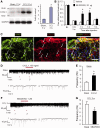
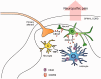
Accumulating evidence suggests that spinal cord astrocytes play an important role in neuropathic pain sensitization by releasing astrocytic mediators (e.g. cytokines, chemokines and growth factors). However, it remains unclear how astrocytes control the release of astrocytic mediators and sustain late-phase neuropathic pain. Astrocytic connexin-43 (now known as GJ1) has been implicated in gap junction and hemichannel communication of cytosolic contents through the glial syncytia and to the extracellular space, respectively. Connexin-43 also plays an essential role in facilitating the development of neuropathic pain, yet the mechanism for this contribution remains unknown. In this study, we investigated whether nerve injury could upregulate connexin-43 to sustain late-phase neuropathic pain by releasing chemokine from spinal astrocytes. Chronic constriction injury elicited a persistent upregulation of connexin-43 in spinal astrocytes for >3 weeks. Spinal (intrathecal) injection of carbenoxolone (a non-selective hemichannel blocker) and selective connexin-43 blockers (connexin-43 mimetic peptides (43)Gap26 and (37,43)Gap27), as well as astroglial toxin but not microglial inhibitors, given 3 weeks after nerve injury, effectively reduced mechanical allodynia, a cardinal feature of late-phase neuropathic pain. In cultured astrocytes, TNF-α elicited marked release of the chemokine CXCL1, and the release was blocked by carbenoxolone, Gap26/Gap27, and connexin-43 small interfering RNA. TNF-α also increased connexin-43 expression and hemichannel activity, but not gap junction communication in astrocyte cultures prepared from cortices and spinal cords. Spinal injection of TNF-α-activated astrocytes was sufficient to induce persistent mechanical allodynia, and this allodynia was suppressed by CXCL1 neutralization, CXCL1 receptor (CXCR2) antagonist, and pretreatment of astrocytes with connexin-43 small interfering RNA. Furthermore, nerve injury persistently increased excitatory synaptic transmission (spontaneous excitatory postsynaptic currents) in spinal lamina IIo nociceptive synapses in the late phase, and this increase was suppressed by carbenoxolone and Gap27, and recapitulated by CXCL1. Together, our findings demonstrate a novel mechanism of astrocytic connexin-43 to enhance spinal cord synaptic transmission and maintain neuropathic pain in the late-phase via releasing chemokines.
Keywords: CXCL1; CXCR2; carbenoxolone (CBX); hemichannels; neuro-glial interaction.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Anand P, Shenoy R, Palmer JE, Baines AJ, Lai RY, Robertson J, et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain. 2011;15:1040–8. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

