On the nature of seizure dynamics
- PMID: 24919973
- PMCID: PMC4107736
- DOI: 10.1093/brain/awu133
On the nature of seizure dynamics
Abstract
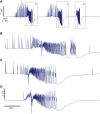
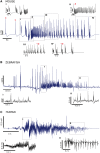
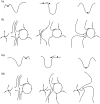
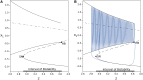
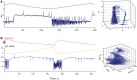
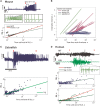
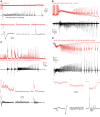
Seizures can occur spontaneously and in a recurrent manner, which defines epilepsy; or they can be induced in a normal brain under a variety of conditions in most neuronal networks and species from flies to humans. Such universality raises the possibility that invariant properties exist that characterize seizures under different physiological and pathological conditions. Here, we analysed seizure dynamics mathematically and established a taxonomy of seizures based on first principles. For the predominant seizure class we developed a generic model called Epileptor. As an experimental model system, we used ictal-like discharges induced in vitro in mouse hippocampi. We show that only five state variables linked by integral-differential equations are sufficient to describe the onset, time course and offset of ictal-like discharges as well as their recurrence. Two state variables are responsible for generating rapid discharges (fast time scale), two for spike and wave events (intermediate time scale) and one for the control of time course, including the alternation between 'normal' and ictal periods (slow time scale). We propose that normal and ictal activities coexist: a separatrix acts as a barrier (or seizure threshold) between these states. Seizure onset is reached upon the collision of normal brain trajectories with the separatrix. We show theoretically and experimentally how a system can be pushed toward seizure under a wide variety of conditions. Within our experimental model, the onset and offset of ictal-like discharges are well-defined mathematical events: a saddle-node and homoclinic bifurcation, respectively. These bifurcations necessitate a baseline shift at onset and a logarithmic scaling of interspike intervals at offset. These predictions were not only confirmed in our in vitro experiments, but also for focal seizures recorded in different syndromes, brain regions and species (humans and zebrafish). Finally, we identified several possible biophysical parameters contributing to the five state variables in our model system. We show that these parameters apply to specific experimental conditions and propose that there exists a wide array of possible biophysical mechanisms for seizure genesis, while preserving central invariant properties. Epileptor and the seizure taxonomy will guide future modeling and translational research by identifying universal rules governing the initiation and termination of seizures and predicting the conditions necessary for those transitions.
Keywords: EEG; bifurcation; epilepsy; modelling; non-linear dynamics.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
On the modelling of seizure dynamics.Brain. 2014 Aug;137(Pt 8):2110-3. doi: 10.1093/brain/awu147. Brain. 2014. PMID: 25057129 Free PMC article.
References
-
- Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci. 1991;101:7–18. - PubMed
-
- Arya R, Kabra M, Gulati S. Epilepsy in children with down syndrome. Epileptic Disord. 2011;13:1–7. - PubMed
-
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131(Pt 7):1818–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

