Ovarian membrane-type matrix metalloproteinases: induction of MMP14 and MMP16 during the periovulatory period in the rat, macaque, and human
- PMID: 24920038
- PMCID: PMC4435413
- DOI: 10.1095/biolreprod.113.115717
Ovarian membrane-type matrix metalloproteinases: induction of MMP14 and MMP16 during the periovulatory period in the rat, macaque, and human
Abstract
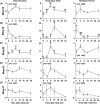
An intrafollicular increase in proteolytic activity drives ovulatory events. Surprisingly, the periovulatory expression profile of the membrane-type matrix metalloproteinases (MT-MMPs), unique proteases anchored to the cell surface, has not been extensively examined. Expression profiles of the MT-MMPs were investigated in ovarian tissue from well-characterized rat and macaque periovulatory models and naturally cycling women across the periovulatory period. Among the six known MT-MMPs, mRNA expression of Mmp14, Mmp16, and Mmp25 was increased after human chorionic gonadotropin (hCG) administration in rats. In human granulosa cells, mRNA expression of MMP14 and MMP16 increased following hCG treatment. In contrast, mRNA levels of MMP16 and MMP25 in human theca cells were unchanged before ovulation but declined by the postovulatory stage. In macaque granulosa cells, hCG increased mRNA for MMP16 but not MMP14. Immunoblotting showed that protein levels of MMP14 and MMP16 in rats increased, similar to their mRNA expression. In macaque granulosa cells, only the active form of the MMP14 protein increased after hCG, unlike its mRNA or the proprotein. By immunohistochemistry, both MMP14 and MMP16 localized to the different ovarian cell types in rats and humans. Treatment with hCG resulted in intense immunoreactivity of MMP14 and MMP16 proteins in the granulosa and theca cells. The present study shows that MMP14 and MMP16 are increased by hCG administration in the ovulating follicle, demonstrating that these MMPs are conserved among rats, macaques, and humans. These findings suggest that MT-MMPs could have an important role in promoting ovulation and remodeling of the ovulated follicle into the corpus luteum.
Keywords: extracellular matrix; granulosa cells; matrix metalloproteinase; ovary; ovulation; theca cell.
© 2014 by the Society for the Study of Reproduction, Inc.
Figures






References
-
- Curry TE, Jr, , Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24:228–241. - PubMed
-
- Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod. 1999;61:14–21. - PubMed
-
- Curry TE, Jr, , Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. - PubMed
-
- Liu YX. Plasminogen activator/plasminogen activator inhibitors in ovarian physiology. Front Biosci. 2004;9:3356–3373. - PubMed
-
- Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–1105. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

