Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence
- PMID: 24920079
- PMCID: PMC4066290
- DOI: 10.1186/1748-717X-9-135
Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence
Abstract
Purpose: To assess the outcome of prostate cancer (PCa) patients diagnosed with oligometastatic disease at recurrence and treated with stereotactic body radiotherapy (SBRT).
Methods: Non-castrate patients with up to 3 synchronous metastases (bone and/or lymph nodes) diagnosed on positron emission tomography - computed tomography, following biochemical recurrence after local curative treatment, were treated with (repeated) SBRT to a dose of 50 Gy in 10 fractions or 30 Gy in 3 fractions. Androgen deprivation therapy-free survival (ADT-FS) defined as the time interval between the first day of SBRT and the initiation of ADT was the primary endpoint. ADT was initiated if more than 3 metastases were detected during follow-up even when patients were still asymptomatic. Secondary endpoints were local control, progression free survival (PFS) and toxicity. Toxicity was scored using the Common Terminology Criteria for Adverse Events.
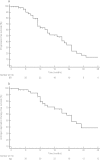
Results: With a median follow-up from time of SBRT of 2 years, we treated 50 patients with 70 metastatic lesions with a local control rate of 100%. The primary involved metastatic sites were lymph nodes (54%), bone (44%), and viscera (2%). The median PFS was 19 mo (95% CI: 13-25 mo) with 75% of recurring patients having ≤3 metastases. A 2nd and 3rd course of SBRT was delivered in 19 and 6 patients respectively. This results in a median ADT-FS of 25 months (20-30 mo). On univariate analysis, only a short PSA doubling time was a significant predictor for both PFS (HR: 0.90, 95% CI: 0.82 - 0.99) and ADT-FS (HR: 0.83; 95% CI: 0.71 - 0.97). Ten patients (20%) developed toxicity following treatment, which was classified as grade I in 7 and grade II in 3 patients.
Conclusion: Repeated SBRT for oligometastatic prostate cancer postpones palliative androgen deprivation therapy with 2 years without grade III toxicity.
Figures


References
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. - DOI - PubMed
-
- Schweizer MT, Zhou XC, Wang H, Yang T, Shaukat F, Partin AW, Eisenberger MA, Antonarakis ES. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Annals of Oncol. 2013;24:2881–2886. doi: 10.1093/annonc/mdt335. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

