A role for the age-dependent loss of α(E)-catenin in regulation of N-cadherin expression and cell migration
- PMID: 24920123
- PMCID: PMC4208646
- DOI: 10.14814/phy2.12039
A role for the age-dependent loss of α(E)-catenin in regulation of N-cadherin expression and cell migration
Abstract
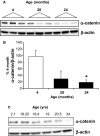
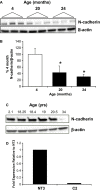
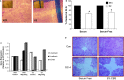
The aging kidney has a decreased ability to repair following acute kidney injury. Previous studies from our laboratory have demonstrated a loss in α-catenin expression in the aging rat kidney. We hypothesize that loss of α-catenin expression in tubular epithelial cells may induce changes that result in a decreased repair capacity. In these studies, we demonstrate that decreased α-catenin protein expression is detectable as early as 20 months of age in male Fischer 344 rats. Protein loss is also observed in aged nonhuman primate kidneys, suggesting that this is not a species-specific response. In an effort to elucidate alterations due to the loss of α-catenin, we generated NRK-52E cell lines with stable knockdown of α(E)-catenin (C2 cells). Interestingly, C2 cells had decreased expression of N-cadherin, decreased cell-cell adhesion, and increased monolayer permeability. C2 had deficits in wound repair, due to alterations in cell migration. Analysis of gene expression in the migrating control cells indicated that expression of N-cadherin and N-CAM was increased during repair. In migrating C2 cells, expression of N-CAM was also increased, but the expression of N-cadherin was not upregulated. Importantly, a blocking antibody against N-cadherin inhibited repair in NRK-52E cells, suggesting an important role in repair. Taken together, these data suggest that loss of α-catenin, and the subsequent downregulation of N-cadherin expression, is a mechanism underlying the decreased migration of tubular epithelial cells that contributes to the inability of the aging kidney to repair following injury.
Keywords: Aging; N‐cadherin; migration; wound repair; α‐catenin.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

Similar articles
-
Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells.Apoptosis. 2015 Jul;20(7):921-9. doi: 10.1007/s10495-015-1129-x. Apoptosis. 2015. PMID: 25894537 Free PMC article.
-
α(E)-catenin regulates BMP-7 expression and migration in renal epithelial cells.Am J Nephrol. 2014;39(5):409-17. doi: 10.1159/000362250. Epub 2014 May 6. Am J Nephrol. 2014. PMID: 24818804 Free PMC article.
-
Loss of N-cadherin and alpha-catenin in the proximal tubules of aging male Fischer 344 rats.Mech Ageing Dev. 2004 Jun;125(6):445-53. doi: 10.1016/j.mad.2004.04.001. Mech Ageing Dev. 2004. PMID: 15178134
-
Fascin2 regulates cisplatin-induced apoptosis in NRK-52E cells.Toxicol Lett. 2017 Jan 15;266:56-64. doi: 10.1016/j.toxlet.2016.11.021. Epub 2016 Dec 15. Toxicol Lett. 2017. PMID: 27989596 Free PMC article.
-
Loss of α(E)-catenin potentiates cisplatin-induced nephrotoxicity via increasing apoptosis in renal tubular epithelial cells.Toxicol Sci. 2014 Sep;141(1):254-62. doi: 10.1093/toxsci/kfu130. Epub 2014 Jun 27. Toxicol Sci. 2014. PMID: 24973089 Free PMC article.
Cited by
-
Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells.Apoptosis. 2015 Jul;20(7):921-9. doi: 10.1007/s10495-015-1129-x. Apoptosis. 2015. PMID: 25894537 Free PMC article.
-
The renoprotective effects of soy protein in the aging rat kidney.Med Res Arch. 2020 Mar;8(3):10.18103/mra.v8i3.2065. doi: 10.18103/mra.v8i3.2065. Epub 2020 Mar 31. Med Res Arch. 2020. PMID: 34222651 Free PMC article.
-
Twist2 Is Upregulated in Early Stages of Repair Following Acute Kidney Injury.Int J Mol Sci. 2017 Feb 10;18(2):368. doi: 10.3390/ijms18020368. Int J Mol Sci. 2017. PMID: 28208580 Free PMC article.
-
Colostrum-Induced Temporary Changes in the Expression of Proteins Regulating the Epithelial Barrier Function in the Intestine.Foods. 2022 Feb 25;11(5):685. doi: 10.3390/foods11050685. Foods. 2022. PMID: 35267318 Free PMC article.
-
MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer.Oncotarget. 2016 Mar 29;7(13):16462-78. doi: 10.18632/oncotarget.7656. Oncotarget. 2016. PMID: 26918943 Free PMC article.
References
-
- Akintola A. D., Crislip Z. L., Catania J. M., Chen G., Zimmer W. E., Burghardt R. C. 2008. Promoter methylation is associated with the age‐dependent loss of N‐cadherin in the rat kidney. Am. J. Physiol. Renal. Physiol.; 294:F170-F176. - PubMed
-
- Alexander N. R., Tran N. L., Rekapally H., Summers C. E., Glackin C., Heimark R. L. 2006. N‐cadherin gene expression in prostate carcinoma is modulated by integrin‐dependent nuclear translocation of twist1. Cancer Res.; 66:3365-3369. - PubMed
-
- Anderson R. J., Ray C. J., Popoff M. R. 2000. Evidence for Rho protein regulation of renal tubular epithelial cell function. Kidney Int.; 58:1996-2006. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

