Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis
- PMID: 24920391
- PMCID: PMC4086274
- DOI: 10.7554/eLife.02882
Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis
Abstract
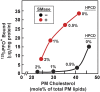
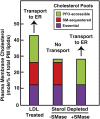
When human fibroblasts take up plasma low density lipoprotein (LDL), its cholesterol is liberated in lysosomes and eventually reaches the endoplasmic reticulum (ER) where it inhibits cholesterol synthesis by blocking activation of SREBPs. This feedback protects against cholesterol overaccumulation in the plasma membrane (PM). But how does ER know whether PM is saturated with cholesterol? In this study, we define three pools of PM cholesterol: (1) a pool accessible to bind 125I-PFO*, a mutant form of bacterial Perfringolysin O, which binds cholesterol in membranes; (2) a sphingomyelin(SM)-sequestered pool that binds 125I-PFO* only after SM is destroyed by sphingomyelinase; and (3) a residual pool that does not bind 125I-PFO* even after sphingomyelinase treatment. When LDL-derived cholesterol leaves lysosomes, it expands PM's PFO-accessible pool and, after a short lag, it also increases the ER's PFO-accessible regulatory pool. This regulatory mechanism allows cells to ensure optimal cholesterol levels in PM while avoiding cholesterol overaccumulation.
Keywords: E. coli; Perfringolysin O; SV-589 human fibroblasts; biochemistry; cell biology; cholesterol–phospholipid complexes; endoplasmic reticulum; lysosomes; sphingomyelin.
Conflict of interest statement
MSB: Reviewing editor,
The other authors declare that no competing interests exist.
Figures
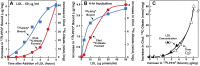
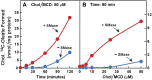
 ) or 0.2 mM sodium [14C]oleate-albumin (7780 dpm/nmol) (
) or 0.2 mM sodium [14C]oleate-albumin (7780 dpm/nmol) ( ). For 125I-PFO* binding (
). For 125I-PFO* binding ( ), after the indicated incubation, the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125I-PFO* (11 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (1.6 μg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation (
), after the indicated incubation, the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125I-PFO* (11 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (1.6 μg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation ( ), after the indicated incubation, the cells were harvested and the increase in content of cholesteryl [14C]oleate was determined after subtraction of the zero-time value (0.09 nmol/mg protein). All values shown are the average of duplicate incubations. (C) Graph showing relation between the increase in 125I-PFO* binding and the increase in cholesteryl [14C]oleate formation. Data taken from (A) and (B). DOI:
), after the indicated incubation, the cells were harvested and the increase in content of cholesteryl [14C]oleate was determined after subtraction of the zero-time value (0.09 nmol/mg protein). All values shown are the average of duplicate incubations. (C) Graph showing relation between the increase in 125I-PFO* binding and the increase in cholesteryl [14C]oleate formation. Data taken from (A) and (B). DOI:

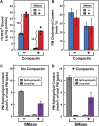
 ) or 0.2 mM sodium [14C]oleate-albumin (4466 dpm/nmol) (
) or 0.2 mM sodium [14C]oleate-albumin (4466 dpm/nmol) ( ), the cells were harvested for assays. For 125IPFO* binding (
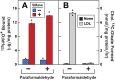
), the cells were harvested for assays. For 125IPFO* binding ( ), after the indicated time the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125IPFO* (45 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (0.4 μg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation (
), after the indicated time the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125IPFO* (45 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (0.4 μg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation ( ), after the indicated time the cells were harvested, and the increase in content of cholesteryl [14C]oleate was determined after subtraction of the zero-time value (0.0 nmol/mg protein). All values shown are the average of duplicate incubations. (B) Effect of SMase treatment of hamster cells on amount of cell surface binding of 125I-PFO*. On day 0, CHO-K1 cells were set up in medium F at 4 × 105 cells per 60-mm dish. On day 1, cells were switched to lipoprotein-deficient medium G. On day 2, cells were treated with fresh medium G containing 50 μM sodium mevalonate in the presence or absence of 10 μM compactin as indicated. On day 3, each monolayer received fresh medium G containing 50 μM mevalonate in the absence or presence of 10 μM compactin and 100 milliunits/ml of SMase as indicated. After incubation for 30 min at 37°C, the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml of ice-cold buffer A containing 25 μg/ml 125I-PFO* (10.5 × 103 cpm/μg). After 2 hr at 4°C, the total amount of cell surface binding of 125I-PFO* was determined. Each bar represents the average of duplicate incubations with individual values shown. Bracketed numbers denote the increase in 125I-PFO* binding resulting from SMase treatment. DOI:
), after the indicated time the cells were harvested, and the increase in content of cholesteryl [14C]oleate was determined after subtraction of the zero-time value (0.0 nmol/mg protein). All values shown are the average of duplicate incubations. (B) Effect of SMase treatment of hamster cells on amount of cell surface binding of 125I-PFO*. On day 0, CHO-K1 cells were set up in medium F at 4 × 105 cells per 60-mm dish. On day 1, cells were switched to lipoprotein-deficient medium G. On day 2, cells were treated with fresh medium G containing 50 μM sodium mevalonate in the presence or absence of 10 μM compactin as indicated. On day 3, each monolayer received fresh medium G containing 50 μM mevalonate in the absence or presence of 10 μM compactin and 100 milliunits/ml of SMase as indicated. After incubation for 30 min at 37°C, the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml of ice-cold buffer A containing 25 μg/ml 125I-PFO* (10.5 × 103 cpm/μg). After 2 hr at 4°C, the total amount of cell surface binding of 125I-PFO* was determined. Each bar represents the average of duplicate incubations with individual values shown. Bracketed numbers denote the increase in 125I-PFO* binding resulting from SMase treatment. DOI:
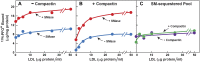

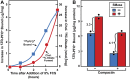
 ) or presence (
) or presence ( ) of 0.2 mM sodium [14C]oleate-albumin (7931 dpm/nmol) for the indicated time. For 125I-PFO* binding (
) of 0.2 mM sodium [14C]oleate-albumin (7931 dpm/nmol) for the indicated time. For 125I-PFO* binding ( ) , after the indicated incubation the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125I-PFO* (10 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (1 µg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation (
) , after the indicated incubation the cells were washed five times as described in ‘Materials and methods’ and then incubated with 2 ml ice-cold buffer A containing 25 μg/ml 125I-PFO* (10 × 103 cpm/μg). After 2 hr at 4°C, the total cell surface binding of 125I-PFO* was determined, and the amount bound after subtraction of the zero-time value (1 µg/mg protein) is plotted as ‘Increase in 125I-PFO* Bound’. For measurement of cholesteryl [14C]oleate formation ( ), the cells were harvested and the content of cholesteryl [14C]oleate was determined. Each value represents the average of duplicate incubations. DOI:
), the cells were harvested and the content of cholesteryl [14C]oleate was determined. Each value represents the average of duplicate incubations. DOI:
References
-
- Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. 2009. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proceedings of the National Academy of Sciences of the United States of America 106:19316–19321. doi: 10.1073/pnas.0910916106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

