Deficits in predictive coding underlie hallucinations in schizophrenia
- PMID: 24920613
- PMCID: PMC4051966
- DOI: 10.1523/JNEUROSCI.0200-14.2014
Deficits in predictive coding underlie hallucinations in schizophrenia
Abstract
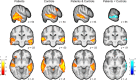
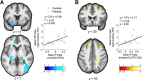
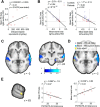
The neural mechanisms that produce hallucinations and other psychotic symptoms remain unclear. Previous research suggests that deficits in predictive signals for learning, such as prediction error signals, may underlie psychotic symptoms, but the mechanism by which such deficits produce psychotic symptoms remains to be established. We used model-based fMRI to study sensory prediction errors in human patients with schizophrenia who report daily auditory verbal hallucinations (AVHs) and sociodemographically matched healthy control subjects. We manipulated participants' expectations for hearing speech at different periods within a speech decision-making task. Patients activated a voice-sensitive region of the auditory cortex while they experienced AVHs in the scanner and displayed a concomitant deficit in prediction error signals in a similar portion of auditory cortex. This prediction error deficit correlated strongly with increased activity during silence and with reduced volumes of the auditory cortex, two established neural phenotypes of AVHs. Furthermore, patients with more severe AVHs had more deficient prediction error signals and greater activity during silence within the region of auditory cortex where groups differed, regardless of the severity of psychotic symptoms other than AVHs. Our findings suggest that deficient predictive coding accounts for the resting hyperactivity in sensory cortex that leads to hallucinations.
Copyright © 2014 the authors 0270-6474/14/348072-11$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
