Parallel midbrain microcircuits perform independent temporal transformations
- PMID: 24920618
- PMCID: PMC4051971
- DOI: 10.1523/JNEUROSCI.4399-13.2014
Parallel midbrain microcircuits perform independent temporal transformations
Abstract
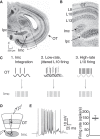
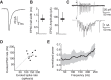
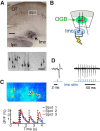
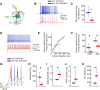
The capacity to select the most important information and suppress distracting information is crucial for survival. The midbrain contains a network critical for the selection of the strongest stimulus for gaze and attention. In avians, the optic tectum (OT; called the superior colliculus in mammals) and the GABAergic nucleus isthmi pars magnocellularis (Imc) cooperate in the selection process. In the chicken, OT layer 10, located in intermediate layers, responds to afferent input with gamma periodicity (25-75 Hz), measured at the level of individual neurons and the local field potential. In contrast, Imc neurons, which receive excitatory input from layer 10 neurons, respond with tonic, unusually high discharge rates (>150 spikes/s). In this study, we reveal the source of this high-rate inhibitory activity: layer 10 neurons that project to the Imc possess specialized biophysical properties that enable them to transform afferent drive into high firing rates (~130 spikes/s), whereas neighboring layer 10 neurons, which project elsewhere, transform afferent drive into lower-frequency, periodic discharge patterns. Thus, the intermediate layers of the OT contain parallel, intercalated microcircuits that generate different temporal patterns of activity linked to the functions of their respective downstream targets.
Keywords: attention; colliculus; decision; inhibition; tectum.
Copyright © 2014 the authors 0270-6474/14/348130-09$15.00/0.
Figures
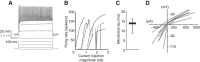

References
-
- Dutar P, Vu HM, Perkel DJ. Multiple cell types distinguished by physiological, pharmacological, and anatomic properties in nucleus HVc of the adult zebra finch. J Neurophysiol. 1998;80:1828–1838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
