Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis
- PMID: 24920620
- PMCID: PMC6608235
- DOI: 10.1523/JNEUROSCI.4415-13.2014
Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis
Abstract
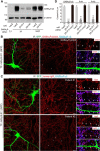
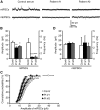
Autoimmune forms of encephalitis have been associated with autoantibodies against synaptic cell surface antigens such as NMDA- and AMPA-type glutamate receptors, GABA(B) receptor, and LGI1. However, it remains unclear how many synaptic autoantigens are yet to be defined. Using immunoproteomics, we identified autoantibodies against the GABA(A) receptor in human sera from two patients diagnosed with encephalitis who presented with cognitive impairment and multifocal brain MRI abnormalities. Both patients had antibodies directed against the extracellular epitope of the β3 subunit of the GABA(A) receptor. The β3-subunit-containing GABA(A) receptor was a major target of the patients' serum antibodies in rat hippocampal neurons because the serum reactivity to the neuronal surface was greatly decreased by 80% when the β3 subunit was knocked down. Our developed multiplex ELISA testing showed that both patients had similar levels of GABA(A) receptor antibodies, one patient also had a low level of LGI1 antibodies, and the other also had CASPR2 antibodies. Application of the patients' serum at the time of symptom presentation of encephalitis to rat hippocampal neuron cultures specifically decreased both synaptic and surface GABA(A) receptors. Furthermore, treatment of neurons with the patients' serum selectively reduced miniature IPSC amplitude and frequency without affecting miniature EPSCs. These results strongly suggest that the patients' GABA(A) receptor antibodies play a central role in the patients' symptoms. Therefore, this study establishes anti-GABA(A) receptor encephalitis and expands the pathogenic roles of GABA(A) receptor autoantibodies.
Keywords: GABAA receptor; autoantibody; autoimmune encephalitis; cognitive impairment; seizure; thymoma.
Copyright © 2014 the authors 0270-6474/14/348151-13$15.00/0.
Figures




References
-
- Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. - DOI - PMC - PubMed
-
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
