Is Parkinson's disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum
- PMID: 24920625
- PMCID: PMC6608236
- DOI: 10.1523/JNEUROSCI.5456-13.2014
Is Parkinson's disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum
Erratum in
- J Neurosci. 2015 Nov 25;35(47):15767
Abstract
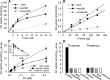
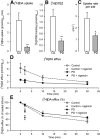
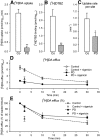
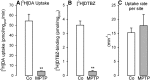
The cause of degeneration of nigrostriatal dopamine (DA) neurons in idiopathic Parkinson's disease (PD) is still unknown. Intraneuronally, DA is largely confined to synaptic vesicles where it is protected from metabolic breakdown. In the cytoplasm, however, free DA can give rise to formation of cytotoxic free radicals. Normally, the concentration of cytoplasmic DA is kept at a minimum by continuous pumping activity of the vesicular monoamine transporter (VMAT)2. Defects in handling of cytosolic DA by VMAT2 increase levels of DA-generated oxy radicals ultimately resulting in degeneration of DAergic neurons. Here, we isolated for the first time, DA storage vesicles from the striatum of six autopsied brains of PD patients and four controls and measured several indices of vesicular DA storage mechanisms. We found that (1) vesicular uptake of DA and binding of the VMAT2-selective label [(3)H]dihydrotetrabenazine were profoundly reduced in PD by 87-90% and 71-80%, respectively; (2) after correcting for DA nerve terminal loss, DA uptake per VMAT2 transport site was significantly reduced in PD caudate and putamen by 53 and 55%, respectively; (3) the VMAT2 transport defect appeared specific for PD as it was not present in Macaca fascicularis (7 MPTP and 8 controls) with similar degree of MPTP-induced nigrostriatal neurodegeneration; and (4) DA efflux studies and measurements of acidification in the vesicular preparations suggest that the DA storage impairment was localized at the VMAT2 protein itself. We propose that this VMAT2 defect may be an early abnormality promoting mechanisms leading to nigrostriatal DA neuron death in PD.
Keywords: Parkinson's disease; VMAT2; dopamine; striatum; synaptic vesicles.
Copyright © 2014 the authors 0270-6474/14/348210-09$15.00/0.
Figures

References
-
- Blesa J, Pifl C, Sánchez-González MA, Juri C, García-Cabezas MA, Adanez R, Iglesias E, Collantes M, Peñuelas I, Sánchez-Hernández JJ, Rodríguez-Oroz MC, Avendaño C, Hornykiewicz O, Cavada C, Obeso JA. The nigrostriatal system in the presymptomatic and symptomatic stages in the MPTP monkey model: a PET, histological and biochemical study. Neurobiol Dis. 2012;48:79–91. doi: 10.1016/j.nbd.2012.05.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
