Chronic GluN2B antagonism disrupts behavior in wild-type mice without protecting against synapse loss or memory impairment in Alzheimer's disease mouse models
- PMID: 24920631
- PMCID: PMC6608240
- DOI: 10.1523/JNEUROSCI.5106-13.2014
Chronic GluN2B antagonism disrupts behavior in wild-type mice without protecting against synapse loss or memory impairment in Alzheimer's disease mouse models
Abstract
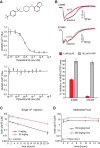
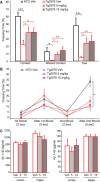
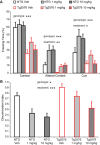
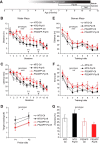
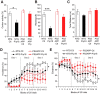
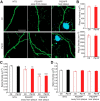
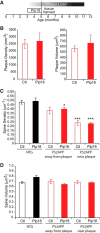
Extensive evidence implicates GluN2B-containing NMDA receptors (GluN2B-NMDARs) in excitotoxic-insult-induced neurodegeneration and amyloid β (Aβ)-induced synaptic dysfunction. Therefore, inhibiting GluN2B-NMDARs would appear to be a potential therapeutic strategy to provide neuroprotection and improve cognitive function in Alzheimer's disease (AD). However, there are no reports of long-term in vivo treatment of AD mouse models with GluN2B antagonists. We used piperidine18 (Pip18), a potent and selective GluN2B-NMDAR antagonist with favorable pharmacokinetic properties, for long-term dosing in AD mouse models. Reduced freezing behavior in Tg2576 mice during fear conditioning was partially reversed after subchronic (17 d) Pip18 treatment. However, analysis of freezing behavior in different contexts indicated that this increased freezing likely involves elevated anxiety or excessive memory generalization in both nontransgenic (NTG) and Tg2576 mice. In PS2APP mice chronically fed with medicated food containing Pip18 for 4 months, spatial learning and memory deficits were not rescued, plaque-associated spine loss was not affected, and synaptic function was not altered. At the same time, altered open field activity consistent with increased anxiety and degraded performance in an active avoidance task were observed in NTG after chronic treatment. These results indicate that long-term treatment with a GluN2B-NMDAR antagonist does not provide a disease-modifying benefit and could cause cognitive liabilities rather than symptomatic benefit in AD mouse models. Therefore, these results challenge the expectation of the therapeutic potential for GluN2B-NMDAR antagonists in AD.
Keywords: Alzheimer's disease; GluN2B; NMDAR; cognition; memory; spine.
Copyright © 2014 the authors 0270-6474/14/348277-12$15.00/0.
Figures

References
-
- Addy C, Assaid C, Hreniuk D, Stroh M, Xu Y, Herring WJ, Ellenbogen A, Jinnah HA, Kirby L, Leibowitz MT, Stewart RM, Tarsy D, Tetrud J, Stoch SA, Gottesdiener K, Wagner J. Single-dose administration of MK-0657, an NR2B-selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson's disease. J Clin Pharmacol. 2009;49:856–864. doi: 10.1177/0091270009336735. - DOI - PubMed
-
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, Zhou H, Kreft AF, Pangalos MN, Sonnenberg-Reines J, Jacobsen JS, Marquis KL. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. - DOI - PMC - PubMed
-
- Costa RO, Lacor PN, Ferreira IL, Resende R, Auberson YP, Klein WL, Oliveira CR, Rego AC, Pereira CM. Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N-methyl-d-aspartate receptor in mature hippocampal cultures treated with amyloid-beta oligomers. Aging Cell. 2012;11:823–833. doi: 10.1111/j.1474-9726.2012.00848.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
