Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy
- PMID: 24920660
- PMCID: PMC4471875
- DOI: 10.1126/scitranslmed.3008008
Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy
Erratum in
- Sci Transl Med. 2014 Nov 5;6(261):261er6
Abstract
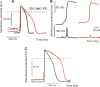
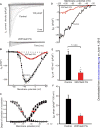
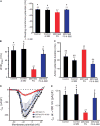
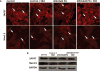
Arrhythmogenic cardiomyopathy (ACM) is characterized by frequent cardiac arrhythmias. To elucidate the underlying mechanisms and discover potential chemical modifiers, we created a zebrafish model of ACM with cardiac myocyte-specific expression of the human 2057del2 mutation in the gene encoding plakoglobin. A high-throughput screen identified SB216763 as a suppressor of the disease phenotype. Early SB216763 therapy prevented heart failure and reduced mortality in the fish model. Zebrafish ventricular myocytes that expressed 2057del2 plakoglobin exhibited 70 to 80% reductions in I(Na) and I(K1) current densities, which were normalized by SB216763. Neonatal rat ventricular myocytes that expressed 2057del2 plakoglobin recapitulated pathobiological features seen in patients with ACM, all of which were reversed or prevented by SB216763. The reverse remodeling observed with SB216763 involved marked subcellular redistribution of plakoglobin, connexin 43, and Nav1.5, but without changes in their total cellular content, implicating a defect in protein trafficking to intercalated discs. In further support of this mechanism, we observed SB216763-reversible, abnormal subcellular distribution of SAP97 (a protein known to mediate forward trafficking of Nav1.5 and Kir2.1) in rat cardiac myocytes expressing 2057del2 plakoglobin and in cardiac myocytes derived from induced pluripotent stem cells from two ACM probands with plakophilin-2 mutations. These observations pinpoint aberrant trafficking of intercalated disc proteins as a central mechanism in ACM myocyte injury and electrical abnormalities.
Copyright © 2014, American Association for the Advancement of Science.
Figures





References
-
- Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. - PubMed
-
- Saffitz JE. The pathobiology of arrhythmogenic cardiomyopathy. Annu Rev Pathol. 2011;6:299–321. - PubMed
-
- Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: An under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. - PubMed
-
- Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

