Unstable reaction intermediates and hysteresis during the catalytic cycle of 5-aminolevulinate synthase: implications from using pseudo and alternate substrates and a promiscuous enzyme variant
- PMID: 24920668
- PMCID: PMC4132793
- DOI: 10.1074/jbc.M114.574731
Unstable reaction intermediates and hysteresis during the catalytic cycle of 5-aminolevulinate synthase: implications from using pseudo and alternate substrates and a promiscuous enzyme variant
Abstract
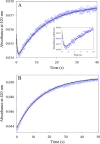
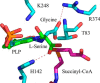
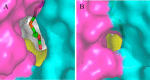
5-Aminolevulinate (ALA), an essential metabolite in all heme-synthesizing organisms, results from the pyridoxal 5'-phosphate (PLP)-dependent enzymatic condensation of glycine with succinyl-CoA in non-plant eukaryotes and α-proteobacteria. The predicted chemical mechanism of this ALA synthase (ALAS)-catalyzed reaction includes a short-lived glycine quinonoid intermediate and an unstable 2-amino-3-ketoadipate intermediate. Using liquid chromatography coupled with tandem mass spectrometry to analyze the products from the reaction of murine erythroid ALAS (mALAS2) with O-methylglycine and succinyl-CoA, we directly identified the chemical nature of the inherently unstable 2-amino-3-ketoadipate intermediate, which predicates the glycine quinonoid species as its precursor. With stopped-flow absorption spectroscopy, we detected and confirmed the formation of the quinonoid intermediate upon reacting glycine with ALAS. Significantly, in the absence of the succinyl-CoA substrate, the external aldimine predominates over the glycine quinonoid intermediate. When instead of glycine, L-serine was reacted with ALAS, a lag phase was observed in the progress curve for the L-serine external aldimine formation, indicating a hysteretic behavior in ALAS. Hysteresis was not detected in the T148A-catalyzed L-serine external aldimine formation. These results with T148A, a mALAS2 variant, which, in contrast to wild-type mALAS2, is active with L-serine, suggest that active site Thr-148 modulates ALAS strict amino acid substrate specificity. The rate of ALA release is also controlled by a hysteretic kinetic mechanism (observed as a lag in the ALA external aldimine formation progress curve), consistent with conformational changes governing the dissociation of ALA from ALAS.
Keywords: 5-Aminolevulinate Synthase; Enzyme Kinetics; Enzyme Mechanism; Heme; Hysteresis; Oxoamine Synthase; Porphyria; Porphyrin; Pyridoxal Phosphate; Sideroblastic Anemia.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Fratz E. J., Stojanovski B. M., Ferreira G. C. (2013) Toward Heme: 5-Aminolevulinate Synthase and Initiation of Porphyrin Synthesis in The Handbook of Porphyrin Science (Ferreira G. C., Kadish K. M., Smith K. M., Guilard R., ed), pp. 1–78, World Scientific Publishing Co., New Jersey
-
- Bottomley S. S. (2004) Sideroblastic Anemias. in Wintrobe's Clinical Hematology (Greer J., Foerster J., Lukens J. N., Rodgers G. M., Paraskevas R., Glader R. B., ed), pp. 1011–1033, Williams and Wilkins, Philadelphia, PA
-
- Whatley S. D., Ducamp S., Gouya L., Grandchamp B., Beaumont C., Badminton M. N., Elder G. H., Holme S. A., Anstey A. V., Parker M., Corrigall A. V., Meissner P. N., Hift R. J., Marsden J. T., Ma Y., Mieli-Vergani G., Deybach J. C., Puy H. (2008) C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am. J. Hum. Genet. 83, 408–414 - PMC - PubMed
-
- Mann S., Ploux O. (2011) Pyridoxal-5′-phosphate-dependent enzymes involved in biotin biosynthesis: structure, reaction mechanism and inhibition. Biochim. Biophys. Acta 1814, 1459–1466 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

