Insight into the architecture of the NuRD complex: structure of the RbAp48-MTA1 subcomplex
- PMID: 24920672
- PMCID: PMC4139204
- DOI: 10.1074/jbc.M114.558940
Insight into the architecture of the NuRD complex: structure of the RbAp48-MTA1 subcomplex
Abstract
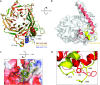
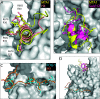
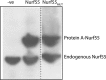
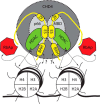
The nucleosome remodeling and deacetylase (NuRD) complex is a widely conserved transcriptional co-regulator that harbors both nucleosome remodeling and histone deacetylase activities. It plays a critical role in the early stages of ES cell differentiation and the reprogramming of somatic to induced pluripotent stem cells. Abnormalities in several NuRD proteins are associated with cancer and aging. We have investigated the architecture of NuRD by determining the structure of a subcomplex comprising RbAp48 and MTA1. Surprisingly, RbAp48 recognizes MTA1 using the same site that it uses to bind histone H4, showing that assembly into NuRD modulates RbAp46/48 interactions with histones. Taken together with other results, our data show that the MTA proteins act as scaffolds for NuRD complex assembly. We further show that the RbAp48-MTA1 interaction is essential for the in vivo integration of RbAp46/48 into the NuRD complex.
Keywords: Chromatin; Chromatin Structure; Gene Regulation; MTA1; NuRD Complex; Protein Assembly; Protein Structure; RBBP4; RbAp48.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

