Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation
- PMID: 24920698
- PMCID: PMC4640193
- DOI: 10.1161/CIRCRESAHA.115.303881
Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation
Abstract
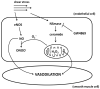
Rationale: Mitochondrial-derived hydrogen peroxide (H2O2) regulates flow-induced dilation (FID) in microvessels from patients with coronary artery disease. The relationship between ceramide, an independent risk factor for coronary artery disease and a known inducer of mitochondrial reactive oxygen species, and FID is unknown.
Objective: We examined the hypothesis that exogenous ceramide induces a switch in the mediator of FID from nitric oxide to H2O2.
Methods and results: Internal diameter changes of resistance arterioles from human adipose and atrial tissue were measured by video microscopy. Mitochondrial H2O2 production was assayed in arterioles using mito peroxy yellow 1. Polyethylene glycol-catalase, rotenone, and Mito-TEMPO impaired FID in healthy adipose arterioles pretreated with ceramide, whereas N(ω)-nitro-l-arginine methyl ester had no effect. Mitochondrial H2O2 production was induced in response to flow in healthy adipose vessels pretreated with ceramide, and this was abolished in the presence of polyethylene glycol-catalase. Immunohistochemistry demonstrated ceramide accumulation in arterioles from both healthy patients and patients with coronary artery disease. N(ω)-nitro-l-arginine methyl ester reduced vasodilation to flow in adipose as well as atrial vessels from patients with coronary artery disease incubated with GW4869, a neutral sphingomyelinase inhibitor, whereas polyethylene glycol-catalase had no effect.
Conclusions: Our data indicate that ceramide has an integral role in the transition of the mediator of FID from nitric oxide to mitochondrial-derived H2O2 and that inhibition of ceramide production can revert the mechanism of dilation back to nitric oxide. Ceramide may be an important target for preventing and treating vascular dysfunction associated with atherosclerosis.
Keywords: ceramides; mitochondria; nitric oxide; reactive oxygen species; sphingomyelin phosphodiesterase.
© 2014 American Heart Association, Inc.
Figures



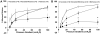

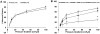
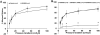
Comment in
-
Ceramide signaling in the coronary microcirculation: a double-edged sword?Circ Res. 2014 Aug 15;115(5):475-7. doi: 10.1161/CIRCRESAHA.114.304589. Circ Res. 2014. PMID: 25124322 Free PMC article. No abstract available.
References
-
- Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. - PubMed
-
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. The international journal of cardiovascular imaging. 2010;26:631–640. - PubMed
-
- Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr., Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: Important role of ca(2+)-activated k(+) channels. Circulation. 2001;103:1992–1998. - PubMed
-
- Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circulation research. 1990;67:529–534. - PubMed
-
- Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. The American journal of physiology. 1991;261:H1706–1715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

