Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide
- PMID: 24920729
- PMCID: PMC4137156
- DOI: 10.1152/ajpregu.00257.2013
Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide
Abstract
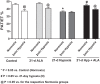
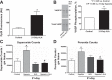
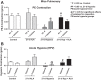
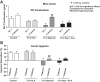
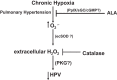
Exposing mice to a chronic hypoxic treatment (10% oxygen, 21 days) that promotes pulmonary hypertension was observed to attenuate the pulmonary vasoconstriction response to acute hypoxia (HPV) both in vivo and in isolated pulmonary arteries. Since catalase restored the HPV response in isolated arteries, it appeared to be attenuated by extracellular hydrogen peroxide. Chronic hypoxia promoted the detection of elevated lung superoxide, extracellular peroxide, extracellular SOD expression, and protein kinase G (PKG) activation [based on PKG dimerization and vasodilator-stimulated phosphoprotein (VASP) phosphorylation], suggesting increased generation of extracellular peroxide and PKG activation may contribute to the suppression of HPV. Aorta from mice exposed to 21 days of hypoxia also showed evidence for extracellular hydrogen peroxide, suppressing the relaxation response to acute hypoxia. Peroxide appeared to partially suppress contractions to phenylephrine used in the study of in vitro hypoxic responses. Treatment of mice with the heme precursor δ-aminolevulinic acid (ALA; 50 mg·kg(-1)·day(-1)) during exposure to chronic hypoxia was examined as a pulmonary hypertension therapy because it could potentially activate beneficial cGMP-mediated effects through promoting a prolonged protoporphyrin IX (PpIX)-elicited activation of soluble guanylate cyclase. ALA attenuated pulmonary hypertension, increases in both superoxide and peroxide, and the suppression of in vitro and in vivo HPV responses. ALA generated prolonged detectible increases in PpIX and PKG-associated phosphorylation of VASP, suggesting PKG activation may contribute to suppression of pulmonary hypertension and prevention of alterations in extracellular peroxide that appear to be attenuating HPV responses caused by chronic hypoxia.
Keywords: aminolevulinic acid; hypoxic pulmonary vasoconstriction; protein kinase G; pulmonary hypertension.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
Roles for redox mechanisms controlling protein kinase G in pulmonary and coronary artery responses to hypoxia.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2295-304. doi: 10.1152/ajpheart.00624.2011. Epub 2011 Sep 16. Am J Physiol Heart Circ Physiol. 2011. PMID: 21926339 Free PMC article.
-
Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2015 Apr 1;308(7):L719-28. doi: 10.1152/ajplung.00155.2014. Epub 2015 Feb 6. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25659899 Free PMC article.
-
Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α.Am J Physiol Lung Cell Mol Physiol. 2014 Feb 15;306(4):L383-91. doi: 10.1152/ajplung.00301.2013. Epub 2013 Dec 27. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24375799 Free PMC article.
-
Oxidant-redox regulation of pulmonary vascular responses to hypoxia and nitric oxide-cGMP signaling.Cardiol Rev. 2010 Mar-Apr;18(2):89-93. doi: 10.1097/CRD.0b013e3181c9f088. Cardiol Rev. 2010. PMID: 20160535 Free PMC article. Review.
-
HIF and pulmonary vascular responses to hypoxia.J Appl Physiol (1985). 2014 Apr 1;116(7):867-74. doi: 10.1152/japplphysiol.00643.2013. Epub 2013 Dec 12. J Appl Physiol (1985). 2014. PMID: 24336881 Free PMC article. Review.
Cited by
-
Hypoxia-Inducible Factor-1α Regulates CD55 in Airway Epithelium.Am J Respir Cell Mol Biol. 2016 Dec;55(6):889-898. doi: 10.1165/rcmb.2015-0237OC. Am J Respir Cell Mol Biol. 2016. PMID: 27494303 Free PMC article.
-
Redox Mechanisms Influencing cGMP Signaling in Pulmonary Vascular Physiology and Pathophysiology.Adv Exp Med Biol. 2017;967:227-240. doi: 10.1007/978-3-319-63245-2_13. Adv Exp Med Biol. 2017. PMID: 29047089 Free PMC article.
-
Renal and Vascular Functional Decline in Aged Low Birth Weight Murine Adults.Kidney Blood Press Res. 2024;49(1):1075-1090. doi: 10.1159/000542141. Epub 2024 Nov 21. Kidney Blood Press Res. 2024. PMID: 39571568
-
Potential role of mitochondrial superoxide decreasing ferrochelatase and heme in coronary artery soluble guanylate cyclase depletion by angiotensin II.Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1439-47. doi: 10.1152/ajpheart.00859.2015. Epub 2016 Apr 1. Am J Physiol Heart Circ Physiol. 2016. PMID: 27037373 Free PMC article.
-
Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia.Am J Physiol Lung Cell Mol Physiol. 2019 Nov 1;317(5):L569-L577. doi: 10.1152/ajplung.00080.2018. Epub 2019 Aug 7. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31389735 Free PMC article.
References
-
- Ahmad M, Kelly MR, Malpani S, Kelly J, Wolin MS. Nox2 derived extracellular superoxide attenuates hypoxic pulmonary vasoconstriction through a novel mechanism in the presence of increased extracellular superoxide dismutase (Abstract). FASEB J 25: 1102.–4., 2011
-
- Alhawaj R, Patel D, Ahmad M, Rémond MC, Leonard Eisenberg LMM, Wolin MS. Treatment of mice with delta-aminolevulinic acid, a generator of the guanylate cyclase activator protoporphyrin IX, prevents the development of hypoxia-induced pulmonary hypertension (Abstract). FASEB J 26: 873.–20., 2012
-
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIα enables oxidant-induced activation. Science 317: 1393–1397, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

