Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs
- PMID: 24920797
- PMCID: PMC4136312
- DOI: 10.1128/JVI.01249-14
Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs
Abstract
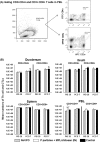
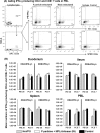
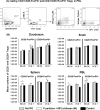
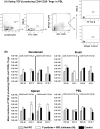
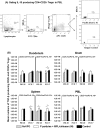
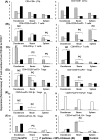
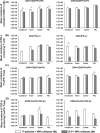
Noroviruses (NoVs) are the leading cause of nonbacterial acute gastroenteritis worldwide in people of all ages. The P particle is a novel vaccine candidate derived from the protruding (P) domain of the NoV VP1 capsid protein. This study utilized the neonatal gnotobiotic pig model to evaluate the protective efficacies of primary infection, P particles, and virus-like particles (VLPs) against NoV infection and disease and the T cell responses to these treatments. Pigs either were vaccinated intranasally with GII.4/1997 NoV (VA387)-derived P particles or VLPs or were inoculated orally with a GII.4/2006b NoV variant. At postinoculation day (PID) 28, pigs either were euthanized or were challenged with the GII.4/2006b variant and monitored for diarrhea and virus shedding for 7 days. The T cell responses in intestinal and systemic lymphoid tissues were examined. Primary NoV infection provided 83% homologous protection against diarrhea and 49% homologous protection against virus shedding, while the P particle and VLP vaccines provided cross-variant protection (47% and 60%, respectively) against diarrhea. The protection rates against diarrhea are significantly inversely correlated with T cell expansion in the duodenum and are positively correlated with T cell expansion in the ileum and spleen. The P particle vaccine primed for stronger immune responses than VLPs, including significantly higher numbers of activated CD4+ T cells in all tissues, gamma interferon-producing (IFN-γ+) CD8+ T cells in the duodenum, regulatory T cells (Tregs) in the blood, and transforming growth factor β (TGF-β)-producing CD4+ CD25- FoxP3+ Tregs in the spleen postchallenge, indicating that P particles are more immunogenic than VLPs at the same dose. In conclusion, the P particle vaccine is a promising vaccine candidate worthy of further development.
Importance: The norovirus (NoV) P particle is a vaccine candidate derived from the protruding (P) domain of the NoV VP1 capsid protein. P particles can be easily produced in Escherichia coli at high yields and thus may be more economically viable than the virus-like particle (VLP) vaccine. This study demonstrated, for the first time, the cross-variant protection (46.7%) of the intranasal P particle vaccine against human NoV diarrhea and revealed in detail the intestinal and systemic T cell responses by using the gnotobiotic pig model. The cross-variant protective efficacy of the P particle vaccine was comparable to that of the VLP vaccine in pigs (60%) and to the homologous protective efficacy of the VLP vaccine in humans (47%). NoV is now the leading cause of pediatric dehydrating diarrhea, responsible for approximately 1 million hospital visits for U.S. children and 218,000 deaths in developing countries. The P particle vaccine holds promise for reducing the disease burden and mortality.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Genotype considerations for virus-like particle-based bivalent norovirus vaccine composition.Clin Vaccine Immunol. 2015 Jun;22(6):656-63. doi: 10.1128/CVI.00015-15. Epub 2015 Apr 22. Clin Vaccine Immunol. 2015. PMID: 25903355 Free PMC article.
-
Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs.Pathogens. 2021 Jul 1;10(7):829. doi: 10.3390/pathogens10070829. Pathogens. 2021. PMID: 34357979 Free PMC article.
-
Functionality and avidity of norovirus-specific antibodies and T cells induced by GII.4 virus-like particles alone or co-administered with different genotypes.Vaccine. 2018 Jan 25;36(4):484-490. doi: 10.1016/j.vaccine.2017.12.009. Epub 2017 Dec 13. Vaccine. 2018. PMID: 29246474
-
Human rotavirus virus-like particle vaccines evaluated in a neonatal gnotobiotic pig model of human rotavirus disease.Expert Rev Vaccines. 2013 Feb;12(2):169-81. doi: 10.1586/erv.13.3. Expert Rev Vaccines. 2013. PMID: 23414408 Review.
-
Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis.Expert Rev Vaccines. 2013 Feb;12(2):155-67. doi: 10.1586/erv.12.145. Expert Rev Vaccines. 2013. PMID: 23414407 Review.
Cited by
-
Norovirus vaccines: Correlates of protection, challenges and limitations.Hum Vaccin Immunother. 2016 Jul 2;12(7):1653-69. doi: 10.1080/21645515.2015.1125054. Epub 2016 Feb 2. Hum Vaccin Immunother. 2016. PMID: 26836766 Free PMC article. Review.
-
Enhanced GII.4 human norovirus infection in gnotobiotic pigs transplanted with a human gut microbiota.J Gen Virol. 2019 Nov;100(11):1530-1540. doi: 10.1099/jgv.0.001336. J Gen Virol. 2019. PMID: 31596195 Free PMC article.
-
Controlled Human Infection Models To Accelerate Vaccine Development.Clin Microbiol Rev. 2022 Sep 21;35(3):e0000821. doi: 10.1128/cmr.00008-21. Epub 2022 Jul 6. Clin Microbiol Rev. 2022. PMID: 35862754 Free PMC article. Review.
-
Antiviral targets of human noroviruses.Curr Opin Virol. 2016 Jun;18:117-25. doi: 10.1016/j.coviro.2016.06.002. Epub 2016 Jun 17. Curr Opin Virol. 2016. PMID: 27318434 Free PMC article. Review.
-
Evaluation of the 50% Infectious Dose of Human Norovirus Cin-2 in Gnotobiotic Pigs: A Comparison of Classical and Contemporary Methods for Endpoint Estimation.Viruses. 2020 Aug 28;12(9):955. doi: 10.3390/v12090955. Viruses. 2020. PMID: 32872283 Free PMC article.
References
-
- Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 368:1121–1130. 10.1056/NEJMsa1206589 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

