West nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression
- PMID: 24920798
- PMCID: PMC4136264
- DOI: 10.1128/JVI.01323-14
West nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression
Abstract
Since its introduction in New York City, NY, in 1999, West Nile virus (WNV) has spread to all 48 contiguous states of the United States and is now the leading cause of epidemic encephalitis in North America. As a member of the family Flaviviridae, WNV is part of a group of clinically important human pathogens, including dengue virus and Japanese encephalitis virus. The members of this family of positive-sense, single-stranded RNA viruses have limited coding capacity and are therefore obligated to co-opt a significant amount of cellular factors to translate their genomes effectively. Our previous work has shown that WNV growth was independent of macroautophagy activation, but the role of the evolutionarily conserved mammalian target of rapamycin (mTOR) pathway during WNV infection was not well understood. mTOR is a serine/threonine kinase that acts as a central cellular censor of nutrient status and exercises control of vital anabolic and catabolic cellular responses such as protein synthesis and autophagy, respectively. We now show that WNV activates mTOR and cognate downstream activators of cap-dependent protein synthesis at early time points postinfection and that pharmacologic inhibition of mTOR (KU0063794) significantly reduced WNV growth. We used an inducible Raptor and Rictor knockout mouse embryonic fibroblast (MEF) system to further define the role of mTOR complexes 1 and 2 in WNV growth and viral protein synthesis. Following inducible genetic knockout of the major mTOR cofactors raptor (TOR complex 1 [TORC1]) and rictor (TORC2), we now show that TORC1 supports flavivirus protein synthesis via cap-dependent protein synthesis pathways and supports subsequent WNV growth.
Importance: Since its introduction in New York City, NY, in 1999, West Nile virus (WNV) has spread to all 48 contiguous states in the United States and is now the leading cause of epidemic encephalitis in North America. Currently, the mechanism by which flaviviruses such as WNV translate their genomes in host cells is incompletely understood. Elucidation of the host mechanisms required to support WNV genome translation will provide broad understanding for the basic mechanisms required to translate capped viral RNAs. We now show that WNV activates mTOR and cognate downstream activators of cap-dependent protein synthesis at early time points postinfection. Following inducible genetic knockout of the major mTOR complex cofactors raptor (TORC1) and rictor (TORC2), we now show that TORC1 supports WNV growth and protein synthesis. This study demonstrates the requirement for TORC1 function in support of WNV RNA translation and provides insight into the mechanisms underlying flaviviral RNA translation in mammalian cells.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


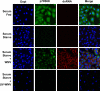

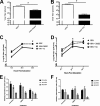
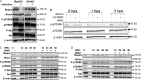
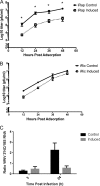

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

