CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge
- PMID: 24920805
- PMCID: PMC4136340
- DOI: 10.1128/JVI.00975-14
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge
Abstract
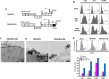
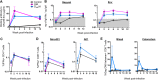
It remains a challenge to develop a successful human immunodeficiency virus (HIV) vaccine that is capable of preventing infection. Here, we utilized the benefits of CD40L, a costimulatory molecule that can stimulate both dendritic cells (DCs) and B cells, as an adjuvant for our simian immunodeficiency virus (SIV) DNA vaccine in rhesus macaques. We coexpressed the CD40L with our DNA/SIV vaccine such that the CD40L is anchored on the membrane of SIV virus-like particle (VLP). These CD40L containing SIV VLPs showed enhanced activation of DCs in vitro. We then tested the potential of DNA/SIV-CD40L vaccine to adjuvant the DNA prime of a DNA/modified vaccinia virus Ankara (MVA) vaccine in rhesus macaques. Our results demonstrated that the CD40L adjuvant enhanced the functional quality of anti-Env antibody response and breadth of anti-SIV CD8 and CD4 T cell responses, significantly delayed the acquisition of heterologous mucosal SIV infection, and improved viral control. Notably, the CD40L adjuvant enhanced the control of viral replication in the gut at the site of challenge that was associated with lower mucosal CD8 immune activation, one of the strong predictors of disease progression. Collectively, our results highlight the benefits of CD40L adjuvant for enhancing antiviral humoral and cellular immunity, leading to enhanced protection against a pathogenic SIV. A single adjuvant that enhances both humoral and cellular immunity is rare and thus underlines the importance and practicality of CD40L as an adjuvant for vaccines against infectious diseases, including HIV-1.
Importance: Despite many advances in the field of AIDS research, an effective AIDS vaccine that can prevent infection remains elusive. CD40L is a key stimulator of dendritic cells and B cells and can therefore enhance T cell and antibody responses, but its overly potent nature can lead to adverse effects unless used in small doses. In order to modulate local expression of CD40L at relatively lower levels, we expressed CD40L in a membrane-bound form, along with SIV antigens, in a nucleic acid (DNA) vector. We tested the immunogenicity and efficacy of the CD40L-adjuvanted vaccine in macaques using a heterologous mucosal SIV infection. The CD40L-adjuvanted vaccine enhanced the functional quality of anti-Env antibody response and breadth of anti-SIV T cell responses and improved protection. These results demonstrate that VLP-membrane-bound CD40L serves as a novel adjuvant for an HIV vaccine.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

