Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance
- PMID: 24920821
- PMCID: PMC4136325
- DOI: 10.1128/JVI.01557-14
Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance
Abstract
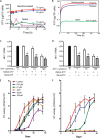
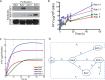
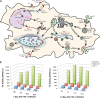
Limitations of antiretroviral therapy (ART) include poor patient adherence, drug toxicities, viral resistance, and failure to penetrate viral reservoirs. Recent developments in nanoformulated ART (nanoART) could overcome such limitations. To this end, we now report a novel effect of nanoART that facilitates drug depots within intracellular compartments at or adjacent to the sites of the viral replication cycle. Poloxamer 407-coated nanocrystals containing the protease inhibitor atazanavir (ATV) were prepared by high-pressure homogenization. These drug particles readily accumulated in human monocyte-derived macrophages (MDM). NanoATV concentrations were ∼1,000 times higher in cells than those that could be achieved by the native drug. ATV particles in late and recycling endosome compartments were seen following pulldown by immunoaffinity chromatography with Rab-specific antibodies conjugated to magnetic beads. Confocal microscopy provided cross validation by immunofluorescent staining of the compartments. Mathematical modeling validated drug-endosomal interactions. Measures of reverse transcriptase activity and HIV-1 p24 levels in culture media and cells showed that such endosomal drug concentrations enhanced antiviral responses up to 1,000-fold. We conclude that late and recycling endosomes can serve as depots for nanoATV. The colocalization of nanoATV at endosomal sites of viral assembly and its slow release sped antiretroviral activities. Long-acting nanoART can serve as a drug carrier in both cells and subcellular compartments and, as such, can facilitate viral clearance.
Importance: The need for long-acting ART is significant and highlighted by limitations in drug access, toxicity, adherence, and reservoir penetrance. We propose that targeting nanoformulated drugs to infected tissues, cells, and subcellular sites of viral replication may improve clinical outcomes. Endosomes are sites for human immunodeficiency virus assembly, and increasing ART concentrations in such sites enhances viral clearance. The current work uncovers a new mechanism by which nanoART can enhance viral clearance over native drug formulations.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Dash P, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RLM, Gelbard H, McMillan J, Gorantla S, Poluektova L. 2012. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1 infected humanized mice. AIDS 26:2135–2144. 10.1097/QAD.0b013e328357f5ad - DOI - PMC - PubMed
-
- Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE. 2010. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. J. Neuroimmune Pharmacol. 5:592–601. 10.1007/s11481-010-9198-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 MH104147/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R01 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

