The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective
- PMID: 24921185
- PMCID: PMC4092857
- DOI: 10.3390/cells3020592
The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective
Abstract
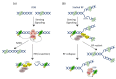
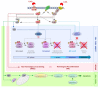
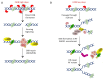
The cytolethal distending toxin (CDT) is produced by many pathogenic Gram-negative bacteria and is considered as a virulence factor. In human cells, CDT exposure leads to a unique cytotoxicity associated with a characteristic cell distension and induces a cell cycle arrest dependent on the DNA damage response (DDR) triggered by DNA double-strand breaks (DSBs). CDT has thus been classified as a cyclomodulin and a genotoxin. Whereas unrepaired damage can lead to cell death, effective, but improper repair may be detrimental. Indeed, improper repair of DNA damage may allow cells to resume the cell cycle and induce genetic instability, a hallmark in cancer. In vivo, CDT has been shown to induce the development of dysplastic nodules and to lead to genetic instability, defining CDT as a potential carcinogen. It is therefore important to characterize the outcome of the CDT-induced DNA damage and the consequences for intoxicated cells and organisms. Here, we review the latest results regarding the host cell response to CDT intoxication and focus on DNA damage characteristics, cell cycle modulation and cell outcomes.
Figures

References
-
- Johnson W.M., Lior H. Response of chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 1987;43:19–23. doi: 10.1111/j.1574-6968.1987.tb02091.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

