Viral oncolysis - can insights from measles be transferred to canine distemper virus?
- PMID: 24921409
- PMCID: PMC4074931
- DOI: 10.3390/v6062340
Viral oncolysis - can insights from measles be transferred to canine distemper virus?
Abstract
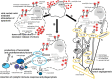
Neoplastic diseases represent one of the most common causes of death among humans and animals. Currently available and applied therapeutic options often remain insufficient and unsatisfactory, therefore new and innovative strategies and approaches are highly needed. Periodically, oncolytic viruses have been in the center of interest since the first anecdotal description of their potential usefulness as an anti-tumor treatment concept. Though first reports referred to an incidental measles virus infection causing tumor regression in a patient suffering from lymphoma several decades ago, no final treatment concept has been developed since then. However, numerous viruses, such as herpes-, adeno- and paramyxoviruses, have been investigated, characterized, and modified with the aim to generate a new anti-cancer treatment option. Among the different viruses, measles virus still represents a highly interesting candidate for such an approach. Numerous different tumors of humans including malignant lymphoma, lung and colorectal adenocarcinoma, mesothelioma, and ovarian cancer, have been studied in vitro and in vivo as potential targets. Moreover, several concepts using different virus preparations are now in clinical trials in humans and may proceed to a new treatment option. Surprisingly, only few studies have investigated viral oncolysis in veterinary medicine. The close relationship between measles virus (MV) and canine distemper virus (CDV), both are morbilliviruses, and the fact that numerous tumors in dogs exhibit similarities to their human counterpart, indicates that both the virus and species dog represent a highly interesting translational model for future research in viral oncolysis. Several recent studies support such an assumption. It is therefore the aim of the present communication to outline the mechanisms of morbillivirus-mediated oncolysis and to stimulate further research in this potentially expanding field of viral oncolysis in a highly suitable translational animal model for the benefit of humans and dogs.
Figures

References
-
- Hedan B., Thomas R., Motsinger-Reif A., Abadie J., Andre C., Cullen J., Breen M. Molecular cytogenetic characterization of canine histiocytic sarcoma: A spontaneous model for human histiocytic cancer identifies deletion of tumor suppressor genes and highlights influence of genetic background on tumor behavior. BMC Cancer. 2011;11:201. doi: 10.1186/1471-2407-11-201. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

